
Write two drawbacks of Rutherford’s atomic model.
Answer
586.8k+ views
Hint: An atomic model is a description of the nature of the constituents of the atom. In Rutherford’s experiment he used an alpha particle emitted from a radioactive particle to go through a thin gold foil, where most of the alpha particles pass through the gold foil without collision and few of them are reflected at a small angle and a very small portion of the alpha particles are deflected back in the incoming direction. Obtain the atomic model from this experiment. From the assumptions of this atomic model we can write the drawbacks.
Complete answer:
With many drawbacks of the watermelon or plum-pudding model of atoms of J. J. Thomson, Rutherford gave a new model of atom. This model is based on the results of the Alpha particle scattering experiment of Rutherford. According to this model he concluded that most of the space in a n atom is empty. The positive charge of the atom is concentrated at the centre of the atom. The volume occupied by the positively charged particle in the atom is very small. This region is called the nucleus of the atom. The electrons move around the nucleus in circular paths. electrons are negatively charged and the nucleus is positively charged. So, they are held together by a strong electrostatic force of attraction.
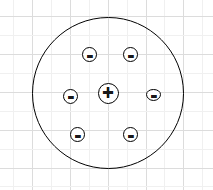
But this model has a few drawbacks or limitations-
A. Rutherford proposed that the electrons revolve around the nucleus in circular orbits. We know that accelerated charge particles emit electromagnetic radiation. Since the electrons move around the nucleus in circular orbit, they are accelerated and hence, they should emit electromagnetic radiation. This emission of electromagnetic radiation will carry out energy from the electron and the radius of the orbit of the electron will become small. At one point the electromagnetic radiation will carry out all its energy and the electron will fall in the nucleus. If this happens the atom will collapse. This is one problem with Rutherford's model.
B. Second, Rutherford didn’t give anything about how the electrons are arranged inside the atom around the nucleus. Hence, we only know that the electrons will revolve around the nucleus but we can’t say how the electrons are arranged in many electron atoms.
Note:
The failures of Rutherford's atomic model are solved by the Bohr model later. He explains that the electrons in an atom revolve in a specific orbit where the electron does not lose energy. So, electrons will never fall to the nucleus. He also described the arrangement of the electrons in these orbits.
Complete answer:
With many drawbacks of the watermelon or plum-pudding model of atoms of J. J. Thomson, Rutherford gave a new model of atom. This model is based on the results of the Alpha particle scattering experiment of Rutherford. According to this model he concluded that most of the space in a n atom is empty. The positive charge of the atom is concentrated at the centre of the atom. The volume occupied by the positively charged particle in the atom is very small. This region is called the nucleus of the atom. The electrons move around the nucleus in circular paths. electrons are negatively charged and the nucleus is positively charged. So, they are held together by a strong electrostatic force of attraction.

But this model has a few drawbacks or limitations-
A. Rutherford proposed that the electrons revolve around the nucleus in circular orbits. We know that accelerated charge particles emit electromagnetic radiation. Since the electrons move around the nucleus in circular orbit, they are accelerated and hence, they should emit electromagnetic radiation. This emission of electromagnetic radiation will carry out energy from the electron and the radius of the orbit of the electron will become small. At one point the electromagnetic radiation will carry out all its energy and the electron will fall in the nucleus. If this happens the atom will collapse. This is one problem with Rutherford's model.
B. Second, Rutherford didn’t give anything about how the electrons are arranged inside the atom around the nucleus. Hence, we only know that the electrons will revolve around the nucleus but we can’t say how the electrons are arranged in many electron atoms.
Note:
The failures of Rutherford's atomic model are solved by the Bohr model later. He explains that the electrons in an atom revolve in a specific orbit where the electron does not lose energy. So, electrons will never fall to the nucleus. He also described the arrangement of the electrons in these orbits.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

The Equation xxx + 2 is Satisfied when x is Equal to Class 10 Maths

Which Country is Called "The Land of Festivals"?

What is Contraception List its four different methods class 10 biology CBSE




