
Why is \[{{\text{N}}_{\text{2}}}\] less reactive at room temperature?
Answer
598.2k+ views
Hint: Before answering the question, we need to have an idea about the structure of \[{{\text{N}}_{\text{2}}}\]. And at the same time the existing chemical bonding between the atoms. Once we get a clear idea, we will be able to answer this question.
Complete step by step solution:
We first will take a look into the chemical bonding of the \[{{\text{N}}_{\text{2}}}\] molecule.
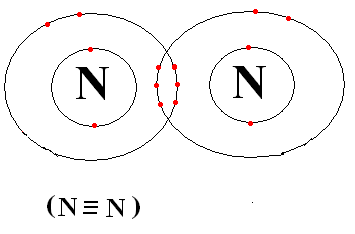 As we can see that there exists a triple bond between the \[{{\text{N}}_{\text{2}}}\] atoms. The triple bond is pretty strong.
As we can see that there exists a triple bond between the \[{{\text{N}}_{\text{2}}}\] atoms. The triple bond is pretty strong.
Therefore, it needs a lot of energy to break those bonds and participate in a reaction. So nitrogen is commonly referred to as an inert gas (more abundance and low-cost).
Dissociation energy of this molecule is fairly high therefore it is less reactive at room temperature.
By Bond Dissociation energy we mean the energy that is required to break a chemical bond. It is one means by quantifying the strength of a chemical bond dissociation energy which is equal to the bond energy, only for diatomic molecules.
The other reason behind the high dissociation energy of nitrogen can also be defined from the fact that nitrogen has six more electrons in a bonding atomic orbital. So its bond order is 3. However, \[{{\text{N}}_{\text{2}}}^{+}\] has only 5 in bonding with bond order is 2.5. Bond dissociation energy is directly proportional to bond order of a molecule so it has more bond dissociation energy.
So, it is due to the high dissociation energy of \[{{\text{N}}_{\text{2}}}\] that it is less reactive at room temperature.
Note: The only way to break the \[{{\text{N}}_{\text{2}}}\] bond is the solution which involves clamshell like organic molecules that holds an atom of the metal hafnium in their opening. Two of these molecules can lock a nitrogen molecule between their metal atoms and break the first two of its bonds.
Although nitrogen makes up 78% of the atmosphere it is not used in many industrial processes as the triple bond is difficult to break.
Complete step by step solution:
We first will take a look into the chemical bonding of the \[{{\text{N}}_{\text{2}}}\] molecule.

Therefore, it needs a lot of energy to break those bonds and participate in a reaction. So nitrogen is commonly referred to as an inert gas (more abundance and low-cost).
Dissociation energy of this molecule is fairly high therefore it is less reactive at room temperature.
By Bond Dissociation energy we mean the energy that is required to break a chemical bond. It is one means by quantifying the strength of a chemical bond dissociation energy which is equal to the bond energy, only for diatomic molecules.
The other reason behind the high dissociation energy of nitrogen can also be defined from the fact that nitrogen has six more electrons in a bonding atomic orbital. So its bond order is 3. However, \[{{\text{N}}_{\text{2}}}^{+}\] has only 5 in bonding with bond order is 2.5. Bond dissociation energy is directly proportional to bond order of a molecule so it has more bond dissociation energy.
So, it is due to the high dissociation energy of \[{{\text{N}}_{\text{2}}}\] that it is less reactive at room temperature.
Note: The only way to break the \[{{\text{N}}_{\text{2}}}\] bond is the solution which involves clamshell like organic molecules that holds an atom of the metal hafnium in their opening. Two of these molecules can lock a nitrogen molecule between their metal atoms and break the first two of its bonds.
Although nitrogen makes up 78% of the atmosphere it is not used in many industrial processes as the triple bond is difficult to break.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




