
Isopropyl chloride and ethyl chloride both react with Na in presence of dry ether. How many products are obtained?
Answer
546.3k+ views
Hint: In Wurtz reaction, two alkyl halide molecules are coupled in presence of sodium metal in anhydrous ether or tetrahydrofuran to form a new carbon bond and thus by giving a symmetrical alkane.
Complete answer:
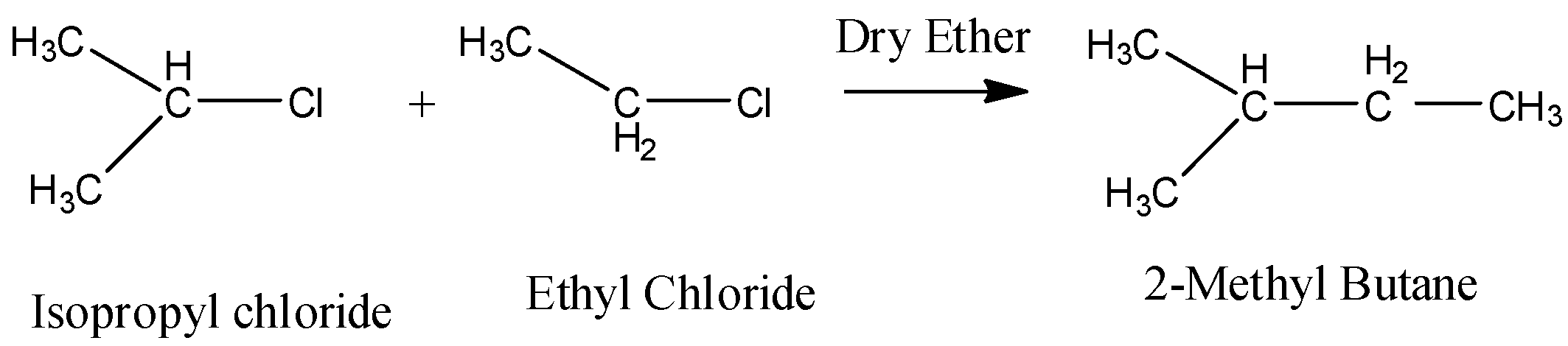
In the question it is given that we have to find the product when isopropyl chloride is going to react with ethyl chloride in the presence of sodium and dry ether.
Let us know what an alkyl halide is.
Alkyl halides (also known as haloalkanes) are compounds in which one or more hydrogen atoms in an alkane have been replaced by halogen atoms. Alkyl halides fall into different classes depending on how the halogen atom is positioned on the chain of carbon atoms. Chloro ethane is also known as Ethyl Chloride. The chemical formula of ethyl chloride is ${ C }_{ 2 }{ H }_{ 5 }Cl$.
When an alkyl halide reacts with Na in presence of dry ether, then free radical alkyl forms along with halide ion because Na is a metal and provides a free electron.
\[2R-X+2Na\xrightarrow{Dry\text{ }Ether}R-R+2NaCl\]
Thus Isopropyl chloride and ethyl chloride reacts with Na in presence of dry ether and gives 2,3-dimethyl butane.
Note:
Methane cannot be synthesized via the Wurtz reaction, since products of an organic coupling reaction must have at least two carbon atoms. The Wurtz coupling method generally fails when tertiary alkyl halides are used.
Complete answer:
In the question it is given that we have to find the product when isopropyl chloride is going to react with ethyl chloride in the presence of sodium and dry ether.
Let us know what an alkyl halide is.
Alkyl halides (also known as haloalkanes) are compounds in which one or more hydrogen atoms in an alkane have been replaced by halogen atoms. Alkyl halides fall into different classes depending on how the halogen atom is positioned on the chain of carbon atoms. Chloro ethane is also known as Ethyl Chloride. The chemical formula of ethyl chloride is ${ C }_{ 2 }{ H }_{ 5 }Cl$.
When an alkyl halide reacts with Na in presence of dry ether, then free radical alkyl forms along with halide ion because Na is a metal and provides a free electron.
\[2R-X+2Na\xrightarrow{Dry\text{ }Ether}R-R+2NaCl\]
Thus Isopropyl chloride and ethyl chloride reacts with Na in presence of dry ether and gives 2,3-dimethyl butane.

Note:
Methane cannot be synthesized via the Wurtz reaction, since products of an organic coupling reaction must have at least two carbon atoms. The Wurtz coupling method generally fails when tertiary alkyl halides are used.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




