
Assume that the nuclear binding energy per nucleus $(B/A)$ versus mass number $(A)$ is as shown in the figure. Use this plot to choose the correct choice(s) given below:
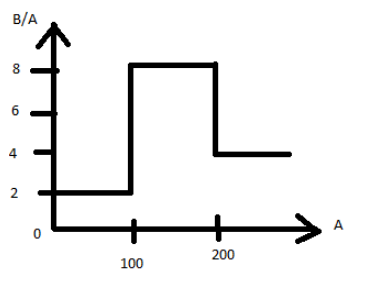
(A) Fusion of two nuclei with mass numbers lying in the range of $1 < A < 50$ will release energy.
(B) Fusion of two nuclei with mass numbers lying in the range of $51 < A < 100$ will release energy.
(C) Fission of a nucleus lying in the mass range of $100 < A < 200$ will release energy when broken into two equal fragments.
(D) Fission of a nucleus lying in the mass range of $200 < A < 260$ will release energy when broken into two equal fragments.

Answer
579.6k+ views
Hint:First let us see a bit about nuclear fusion and fission:
The splitting of an atom into two us fission and the combining of two lighter atoms into one is fusion. They are totally different processes.Both fission and fusion are power-producing nuclear reactions, but the methods are not the same. Fission is the separation into the lighter nuclei of a heavy, unstable nucleus and fusion is the phase in which two light nuclei fuse together, producing large quantities of energy. Fission is used because it can be controlled in nuclear power reactors, although fusion is not used to generate power because the reaction is not readily controlled and it is costly to establish the necessary conditions.
Complete step by step answer:
The fact that there is a higher bonding energy in the region between $A = 100 - 200$
means that if the atoms produced lies in this area of equilibrium, then the breakup of heavier nuclei or the mixture of lighter nuclei will produce nuclei that are more closely bound.Two atoms with atomic mass number between $50 - 100$ will interact to generate an atomic number with $100 - 200$ for fusion and will be stable by the release of energy than individual atoms.Hence, option B is correct.
For fission atoms with atomic mass number more than $200 - 260$, two atoms with equal atomic number between $100 - 200$ will form, and by releasing the energy, new atoms will be more stable than the parent atom.
Hence, option D is also correct.
Note:Here we have to carefully observe the atomic numbers range in the graph provided. The stability of the fission and fusion reaction will depend on this graph which also gives us the nuclear binding energy.
The splitting of an atom into two us fission and the combining of two lighter atoms into one is fusion. They are totally different processes.Both fission and fusion are power-producing nuclear reactions, but the methods are not the same. Fission is the separation into the lighter nuclei of a heavy, unstable nucleus and fusion is the phase in which two light nuclei fuse together, producing large quantities of energy. Fission is used because it can be controlled in nuclear power reactors, although fusion is not used to generate power because the reaction is not readily controlled and it is costly to establish the necessary conditions.
Complete step by step answer:
The fact that there is a higher bonding energy in the region between $A = 100 - 200$
means that if the atoms produced lies in this area of equilibrium, then the breakup of heavier nuclei or the mixture of lighter nuclei will produce nuclei that are more closely bound.Two atoms with atomic mass number between $50 - 100$ will interact to generate an atomic number with $100 - 200$ for fusion and will be stable by the release of energy than individual atoms.Hence, option B is correct.
For fission atoms with atomic mass number more than $200 - 260$, two atoms with equal atomic number between $100 - 200$ will form, and by releasing the energy, new atoms will be more stable than the parent atom.
Hence, option D is also correct.
Note:Here we have to carefully observe the atomic numbers range in the graph provided. The stability of the fission and fusion reaction will depend on this graph which also gives us the nuclear binding energy.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




