




Stepwise Laboratory Preparation Methods for Aldehydes and Ketones
The Methods Of Preparation Of Aldehydes And Ketones form a core concept in JEE Main organic chemistry. These compounds, called carbonyls, contain a carbon-oxygen double bond (C=O) and are vital intermediates for both laboratory syntheses and large-scale chemical manufacturing. Their systematic preparation is essential for mastering organic transformations and scoring in JEE Main questions on functional group interconversions and mechanisms.
Aldehydes (R-CHO) and ketones (R-CO-R′) differ by the location of their carbonyl group: aldehydes have it at the chain end, while ketones have it within the carbon skeleton. Both find widespread application in polymers, pharmaceuticals, fragrances, and as starting materials in organic synthesis. Recognizing their preparation routes allows quick analysis and recall for competitive exams.
Classification of Preparation Methods for Aldehydes and Ketones
The main methods of preparation of aldehydes and ketones fall into three practical categories. Understanding this classification aids memory and facilitates accurate MCQ attempts on reaction types or reagents.
- Laboratory methods – small-scale, using pure reagents for mechanism study and analysis.
- Industrial (commercial) methods – used for bulk synthesis via economical reagents.
- Special/reductive methods – targeted for selectivity and functional-group specificity.
The below table summarises the main approaches at a glance for revision:
| Method | Aldehydes | Ketones |
|---|---|---|
| Oxidation of Alcohols | Primary alcohols → aldehydes (PCC, mild oxidants) | Secondary alcohols → ketones (K2Cr2O7, Jones) |
| Distillation with Metal Salts | Calcium salt of formic acid + CaO | Calcium salt of fatty acids + CaO |
| Reduction Reactions | Rosenmund, Stephen’s, Gattermann-Koch | Friedel-Crafts acylation, dry distillation |
| Hydration of Alkynes | HC≡CH + H2O (HgSO4/H2SO4) | R–C≡C–R′ + H2O (catalyst) |
| Ozonolysis of Alkenes | Alkene with terminal double bond | Disubstituted alkene |
| Commercial (Industrial) | Hydroformylation (oxo process) | Oxidation of hydrocarbons, air-oxidation |
Laboratory Preparation of Aldehydes
Several routes exist for synthesising aldehydes in the lab, each with signature reagents and mechanisms tested in JEE Main:
- Oxidation of primary alcohols: RCH2OH + PCC (Pyridinium chlorochromate) or K2Cr2O7/H2SO4 yields RCHO; mild oxidants prevent over-oxidation to acids.
- Rosenmund Reduction: Acid chloride (RCOCl) reduced to aldehyde (RCHO) using hydrogen gas over Pd/BaSO4 (selective and prevents further reduction to alcohol).
- Stephen’s Reduction: Nitrile (RCN) reduced to imine → hydrolysed to aldehyde using stannous chloride (SnCl2/HCl, followed by H2O).
- Distillation of calcium formate: Ca(HCOO)2 + CaO on heating yields formaldehyde (HCHO).

Specialised methods, such as Gattermann-Koch (aromatic aldehyde from benzene + CO/HCl, AlCl3/CuCl) and hydrolysis of geminal dihalides, have specific JEE relevance for aromatic systems and are often basis for MCQs.
Industrial and Commercial Preparation Methods
For large-scale synthesis, Methods Of Preparation Of Aldehydes And Ketones are optimised for yield and economy. JEE Main often tests the names and outline reactions of these commercial methods:
- Hydroformylation (Oxo Process): Alkene + CO + H2 (Rh or Co catalyst) yields aldehyde. Example: Propene → butanal.
- Wacker Process: Converts alkenes to methyl ketones by oxidation with PdCl2/CuCl2, e.g., ethene to acetaldehyde.
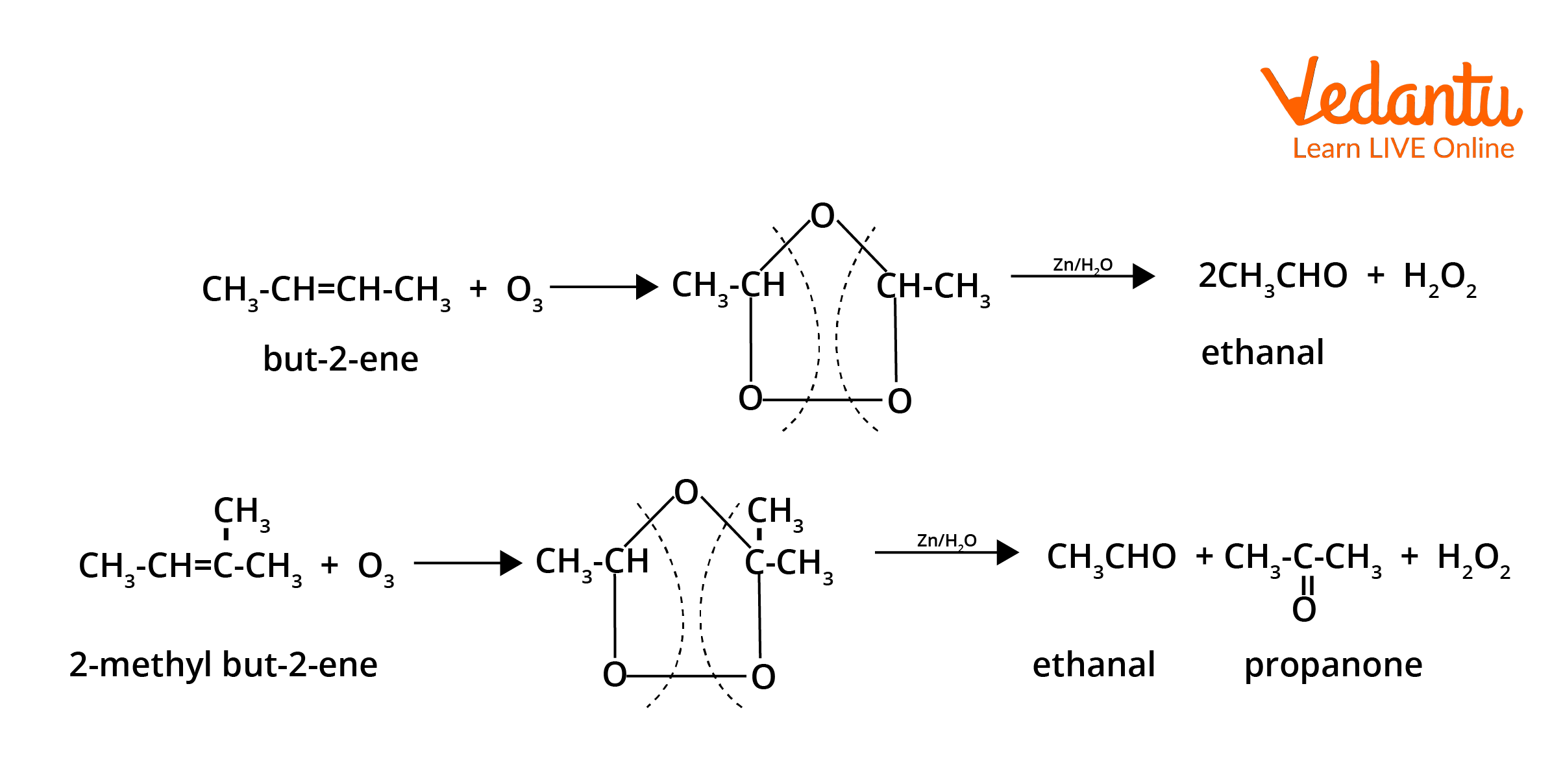
- Ozonolysis of alkenes: Cleaves C=C to yield aldehydes or ketones after reductive workup (Zn/H2O).
- Air oxidation of hydrocarbons: e.g., cumene to acetone and benzaldehyde by catalytic oxidation.
Laboratory Preparation of Ketones
Preparation of ketones for JEE Main focuses on reactions that introduce the carbonyl group within a chain. The key preparative routes include:
- Oxidation of secondary alcohols: R1CH(OH)R2 + K2Cr2O7/H2SO4 or Jones reagent yields R1CO–R2. No over-oxidation risk (no α-hydrogen loss).
- Dry distillation of calcium salts: Calcium salt of carboxylic acids with CaO offers symmetrical ketones, e.g., calcium acetate to acetone.
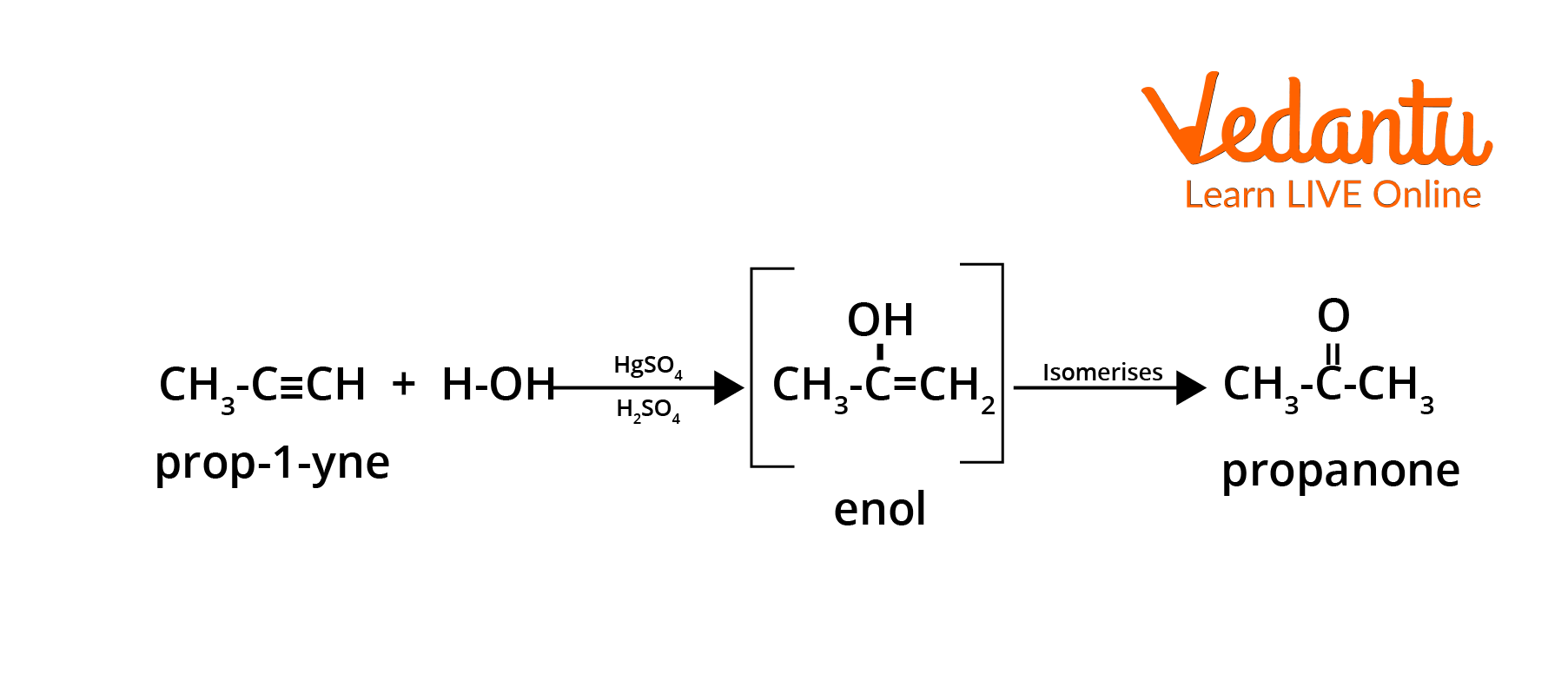
- Hydration of alkynes: Internal alkynes on hydration (via HgSO4/H2SO4) form ketones; for terminal alkynes, methyl ketones form (Markovnikov addition).
- Friedel-Crafts acylation: Aromatic hydrocarbons react with acyl chlorides and AlCl3 catalyst to yield aryl ketones (no reaction for deactivated rings).
Key Reagents and Their Roles in Carbonyl Compound Synthesis
Each preparation uses signature reagents. The right reagent often acts as a clue in direct MCQs. Revise the essentials:
| Compound | Reagent/Condition | Function |
|---|---|---|
| Aldehyde (RCHO) | PCC, Rosenmund, SnCl2/HCl, CO + HCl + AlCl3 | Selective mild oxidation or reduction |
| Ketone (RCOR) | K2Cr2O7, Friedel-Crafts, HgSO4 | Chain oxidation, acylation, hydration |
| Both (RCHO & RCOR) | Ozonolysis, Ca salts dry distillation | Double bond cleavage |
Tips for Mastering Aldehyde and Ketone Preparation
- Always pair primary alcohols with aldehydes, and secondary alcohols with ketones. Do not confuse oxidant strength.
- Recognise calcium salt distillation forms symmetrical products only. Asymmetry requires mixed salts but with lower yield.
- Buffers (BaSO4) in Rosenmund reduction prevent over-reduction; highlight this for mechanism-type questions.
- External ligands (AlCl3, ZnCl2) are key activators for acylation and Gattermann methods, especially in aromatic conversions.
- Treat air-oxidation and hydroformylation as “industrial” in exam context – laboratory parallels aren’t direct.
- Remember that ketones are not easily oxidised further; aldehydes are susceptible to both mild and strong oxidants.
- For direct MCQs, histrionic reagents like PCC, SnCl2, or HgSO4/H2SO4 help eliminate wrong options.
Linking Preparation with Analysis and Applications
Being proficient in methods of preparation of aldehydes and ketones enables fast identification in JEE Main problems involving sequence transformations, functional group conversions, or mechanism prediction. For deeper study:
- Review structure-property relations at Difference Between Aldehydes and Ketones.
- Read carbonyl group reaction trends via Organic Compounds Containing Oxygen.
- Practise recognizing precursor alcohols and oxygenation with Preparation of Haloalkane from Alcohol.
- For more JEE-style mechanisms, visit Clemmensen and Wolff Kishner Reduction.
- Use Vedantu’s revision sheets and notes available under JEE Chemistry Revision Notes for last-minute review.
Mastering these techniques will help you score well on all functional group transformation problems in the JEE Main Chemistry section.
FAQs on Preparation Methods of Aldehydes and Ketones: Lab and Industrial Techniques
1. What is the method of preparation of aldehydes and ketones?
Aldehydes and ketones can be prepared using several laboratory and industrial methods, each important for exams and practicals. Main preparation methods include:
- Oxidation of primary alcohols (for aldehydes) and secondary alcohols (for ketones)
- Rosenmund reduction (for aldehydes only)
- Hydration of alkynes
- Ozonolysis of alkenes
- Hydroformylation (industrial method for aldehydes)
2. What is the laboratory method of preparation of ketones?
The main laboratory method to prepare ketones is the oxidation of secondary alcohols using controlled reagents. Key steps include:
- Oxidation of secondary alcohols (e.g., isopropanol to acetone) using PCC, K2Cr2O7, or acidified KMnO4
- Hydration of alkynes (using dilute H2SO4 and HgSO4) to yield methyl ketones
- Ozonolysis of alkenes can also yield ketones, especially from unsymmetrical alkenes
3. What are the two tests for detecting aldehydes?
The two classic tests for aldehydes are Tollens’ test and Fehling’s test, both used to identify aldehyde functional groups:
- Tollens’ Test: Aldehydes reduce ammoniacal silver nitrate to metallic silver, producing a silver mirror.
- Fehling’s Test: Aldehydes reduce Fehling’s solution to a red precipitate of Cu2O.
4. What is the commercial method of preparation of aldehydes?
Commercially, aldehydes are most often prepared by large-scale processes targeting industrial efficiency.
- Hydroformylation (Oxo process): Alkenes react with CO and H2 under cobalt or rhodium catalysts to form aldehydes.
- Partial oxidation of alcohols is used for aromatic aldehydes (e.g., formaldehyde from methanol).
- Rosenmund reduction (for aliphatic aldehydes), using hydrogenation of acyl chlorides over Pd/BaSO4.
5. Which reagents convert alkyne to aldehyde?
Alkynes are converted to aldehydes using hydroboration-oxidation reactions, particularly for terminal alkynes.
- Reagents: 1) Disiamylborane (Sia2BH) or BH3 for hydroboration, 2) H2O2/NaOH for oxidation
- Terminal alkynes yield aldehydes, while internal alkynes give ketones
6. What are the key differences in preparation methods of aldehydes and ketones?
The principal differences are based on the types of alcohols oxidized and the reagents used.
- Aldehydes: Prepared from primary alcohols oxidation or via Rosenmund reduction, risk over-oxidation to acids, require milder oxidants (e.g., PCC)
- Ketones: Prepared from secondary alcohols oxidation, via hydration of alkynes, and not easily oxidized further
- Alkynes yield aldehydes using hydroboration-oxidation, but ketones via acid-catalyzed hydration
7. Can ketones be prepared directly from primary alcohols?
No, ketones cannot be prepared directly from primary alcohols. Only secondary alcohols can be oxidized to ketones, while primary alcohols yield aldehydes (or carboxylic acids upon over-oxidation).
8. Which are the main laboratory reagents used for preparation of aldehydes and ketones?
Common laboratory reagents for preparing aldehydes and ketones include:
- Pyridinium chlorochromate (PCC) - selective for producing aldehydes from primary alcohols
- Potassium dichromate (K2Cr2O7)/Sulphuric acid - for oxidation
- Hydrogen gas + Pd/BaSO4 (Rosenmund reduction for aldehydes)
- Disiamylborane (Sia2BH) and H2O2/NaOH (for hydroboration-oxidation)
- HgSO4 in H2SO4 - hydration of alkynes (to ketones)
9. Do all aldehyde preparation methods work for aromatic compounds?
No, not every method applies equally to aromatic compounds. For example:
- Oxidation of methyl side-chains (toluene) yields aromatic aldehydes like benzaldehyde
- Rosenmund reduction, ozonolysis, and Gattermann–Koch formylation are commonly used for aromatic systems
- Some aliphatic methods (like simple PCC oxidation of primary alcohols) are less practical for aromatic aldehydes
10. What are common mistakes to avoid when preparing aldehydes and ketones?
Avoiding these common mistakes will help ensure correct exam answers:
- Do not use strong oxidants for aldehyde prep—risk of forming carboxylic acids
- Remember primary alcohols → aldehydes, secondary alcohols → ketones
- Terminal alkynes via acid hydration produce ketones, not aldehydes
- Tollens’ and Fehling’s tests: Only for aldehydes, not ketones
- Incorrect reagent or over-oxidation commonly leads to wrong products
































