




What Are the Main Components of an Atom?
Have you ever wondered how everything we see is made up of? Look at your surroundings. You can see buildings and roads. Buildings are made by joining bricks. Bricks are the fundamental unit of the building. Similarly, all the matter (that occupies space) is made up of atoms.
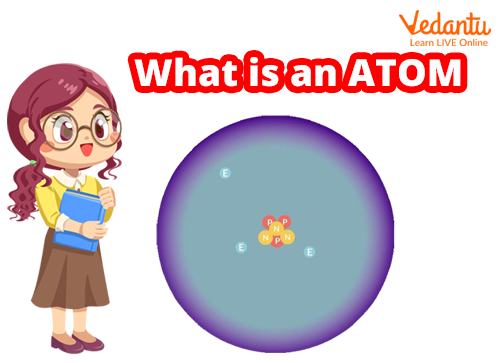
Basic Structure of Atom
Discovery of an Atom
Democritus, a Greek philosopher, introduced the concept of the atom around 450 BC. His model was solid, and it stated that all atoms vary in size, mass, shape, position, and arrangement. In 1800, John Dalton reintroduced the atom and proposed that all matter was made up of atoms, which were indivisible and indestructible building blocks. Rutherford provided the final structure in 1911.
What is an Atom?
Atoms are invisible, but they make up everything around you. Atom's simple definition states that atoms are the building blocks of matter. Each type of atom contributes to the formation of a pure substance known as an element. You've probably heard of oxygen, lead, gold, etc. These are all elements, after all. A pure gold necklace is composed of billions of gold atoms.
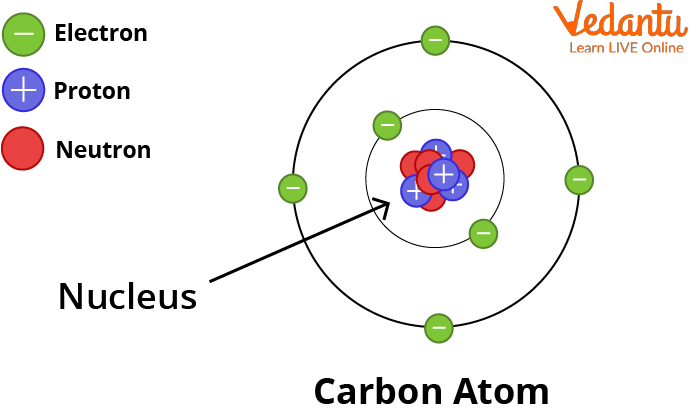
The atom is the fundamental unit of matter in the universe. Atoms are extremely small particles that are made up of even smaller particles. Electrons, protons, and neutrons are the fundamental particles that make up an atom. Atoms combine with other atoms to form matter. Anything is made up of a lot of atoms. There are so many atoms in a single human body that we won't even attempt to count them.
Atoms are classified according to the number of electrons, protons, and neutrons they contain. An element is made up of various types of atoms. There are 118 when known elements.
Structure of an Atom: How Atoms are Formed
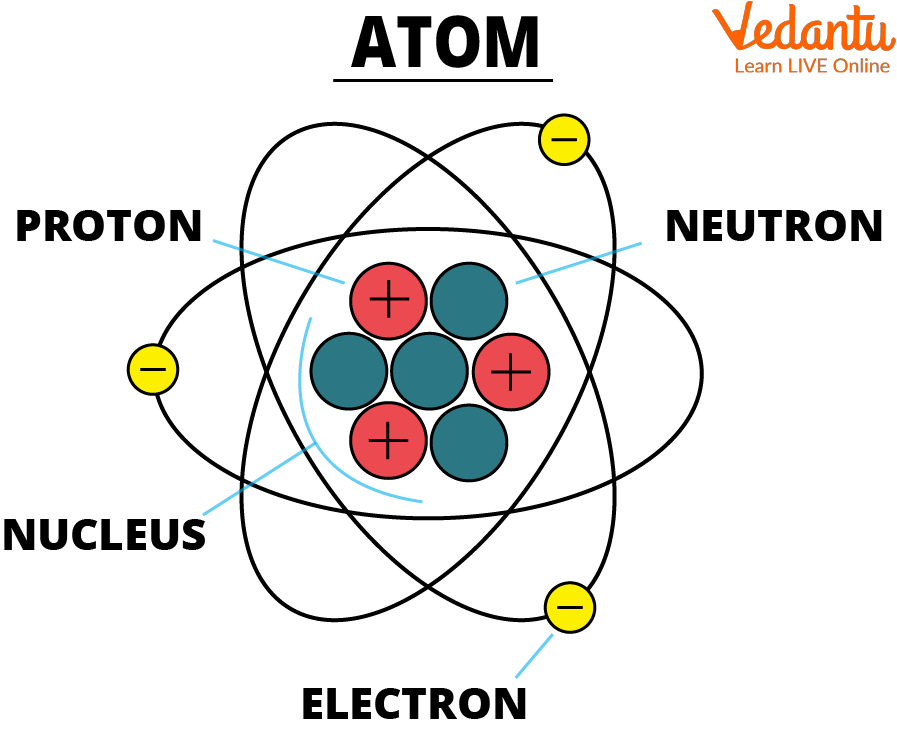
Atom
See, the atom diagram, you can observe that an atom is made up of even smaller particles. Even though, atoms are the smallest unit of matter it still contains sub-atomic particles within it that are:
Proton
Neutron
Electron
Protons and Neutrons Combine To Form the Nucleus.
Subatomic Particles of an Atom
The Proton
The proton is a positively charged particle that is located in the nucleus of the atom. The hydrogen atom is unique in that its nucleus contains only one proton and no neutron. The number of protons in an atom determines the element. This is referred to as the element's atomic number. Ernest Rutherford, a physicist from New Zealand, discovered protons in the early 1900s.
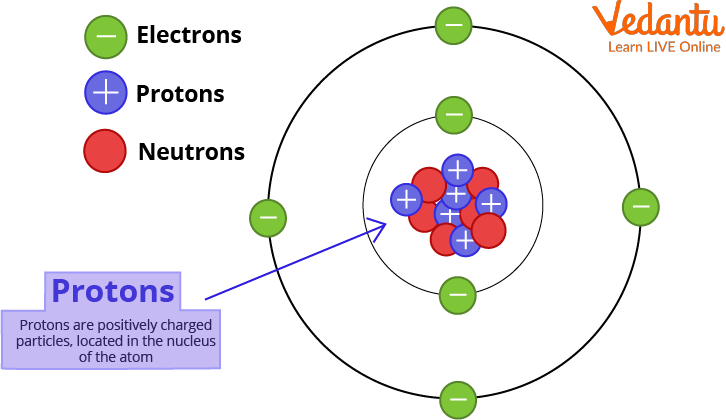
Proton
The Electron
The electron is a negatively charged particle that revolves around the nucleus. Because electrons spin so fast around the nucleus, scientists can never be certain of their exact location. When an atom has the same number of electrons and protons, it is said to be neutrally charged.
The positive charge of the protons attracts electrons to the nucleus. Neutrons and protons are much smaller than electrons. Approximately 1800 times smaller!
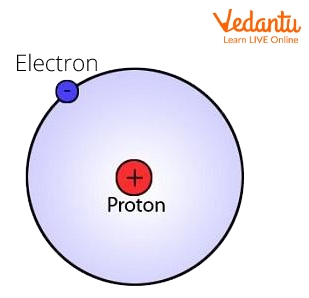
Electron
The Neutron
A neutron is an atom's neutral nucleus with no electric charge and a slightly larger mass than a proton.
The number of neutrons in an atom influences its mass and radioactivity. It was discovered by James Chadwick, a British physicist.
Neutron
Fun Facts About Atom
Quark - The quark is a minute particle that makes up neutrons and protons. Quarks are almost difficult to identify and it's only recently that researchers sorted out they existed. They were founded in 1964 by Murray Gell-Mann. There are 6 sorts of quarks: top, bottom, up, down, strange, and charm.
Neutrino - Neutrinos are shaped by atomic responses. They are like electrons with practically no charge and are typically going at the speed of light. A great many neutrinos are radiated by the sun consistently. Neutrinos go directly through most solids including people!
So far, you have seen the journey of the atom and its properties.
Let's summarise:
Atoms are the basic structure blocks for all matter.
It comprises protons, neutrons, and electrons.
Protons are positively charged.
Electrons are negatively charged.
Neutrons are neutral in charge.
Protons and neutrons combinedly form the nucleus.
FAQs on Basic Structure of Atom: Definition, Parts & Easy Explanation
1. What is the basic structure of an atom?
An atom has a central core called the nucleus, which contains positively charged particles called protons and neutral particles called neutrons. Orbiting this nucleus are negatively charged particles called electrons, which move in specific paths or shells. Most of the atom's mass is in the tiny, dense nucleus, while the electrons make up most of its volume.
2. What are the three main particles inside an atom and where are they located?
The three main subatomic particles and their locations are:
- Protons: These have a positive charge and are found inside the nucleus at the centre of the atom.
- Neutrons: These have no charge (they are neutral) and are also located inside the nucleus with the protons.
- Electrons: These have a negative charge and orbit the nucleus in different energy levels or shells.
3. If atoms are mostly empty space, why don't we fall through the floor?
This is because of the powerful repulsive forces between electrons. The electrons in the atoms of your feet repel the electrons in the atoms of the floor very strongly. This force between the electron clouds is what creates the feeling of solidity and prevents you from passing through solid objects, even though the atoms themselves are mostly empty.
4. What is the difference between an atom’s atomic number and mass number?
The main difference is what they count inside an atom:
- The atomic number tells you the number of protons. This number is unique for each element and defines what element it is. For example, any atom with 6 protons is carbon.
- The mass number is the total count of protons and neutrons added together in the nucleus. It gives an idea of the atom's total mass.
In short, atomic number identifies the element, while mass number measures its mass.
5. Why are electrons so important for understanding how an atom behaves?
Electrons, particularly the ones in the outermost shell (called valence electrons), determine an atom's chemical properties. An atom's tendency to gain, lose, or share these electrons is what drives chemical bonding and all chemical reactions. The arrangement of electrons explains why some elements are very reactive while others are stable and non-reactive.
6. Do individual atoms have a colour?
No, individual atoms are too small to have a colour on their own. Colour is a property that arises from how a material reflects light. When billions of atoms of the same type group together to form an element, like copper or gold, their collective structure interacts with light to produce the colour we see.
7. What are some simple facts about atoms?
Here are a few key facts about atoms:
- They are incredibly tiny: You could fit millions of atoms across the width of a single human hair.
- They are ancient: The atoms that make up everything around us, including our bodies, were formed billions of years ago in stars.
- They are the building blocks: Every object in the universe, whether solid, liquid, or gas, is made up of atoms.
- The number of protons defines them: An atom with one proton is always hydrogen, and an atom with eight protons is always oxygen.









