Essential vs Non-Essential Amino Acids: Key Differences and Roles
This article explains what amino acids are and explores the different types of amino acids including the complete amino acids list of the 20 amino acids that are fundamental to life. Whether you are wondering how many amino acids are there or seeking to understand their amino acid structure, you’ll find clear, student-friendly explanations below.
Overview
They are organic compounds that serve as the building blocks of proteins. Every protein in our body is made up of long chains of amino acids, making them essential for growth, repair, and overall biological function. In simple terms, what are amino acids? They are molecules that contain both an amino group (–NH₂) and a carboxyl group (–COOH) attached to a central carbon. This fundamental concept is crucial to understanding how proteins are assembled.
General Properties of Amino Acids
High Melting/Boiling Points: Due to their ionic nature, amino acids have high melting and boiling points.
Crystalline Solids: They typically appear as white, crystalline solids.
Taste and Solubility: Some amino acids are sweet, others tasteless or bitter. Most are water-soluble but insoluble in many organic solvents.
Zwitterionic Form: In aqueous solutions, the amino acids structure commonly exists as a zwitterion—meaning the molecule carries both a positive and a negative charge.
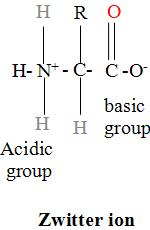
Classification of Amino Acids
Essential and Non-Essential Amino Acids
The classification of amino acids is based on the body’s ability to synthesise them:
Essential Amino Acids: These cannot be made by our bodies and must be obtained from our diet. Our essential amino acids list includes:
Isoleucine, Histidine, Lysine, Leucine, Phenylalanine, Tryptophan, Methionine, Threonine, and Valine.
Non-Essential Amino Acids: These are produced by the human body. Examples include:
Alanine, Asparagine, Arginine, Aspartic acid, Glutamic acid, Cysteine, Glutamine, Proline, Glycine, Serine, and Tyrosine.
Read More: Difference Between Essential and Non-Essential Amino Acids
Amino Acids Structure
The amino acid structure is remarkably simple yet elegant. All types of amino acids share a common backbone:
H₂N–CH(R)–COOH
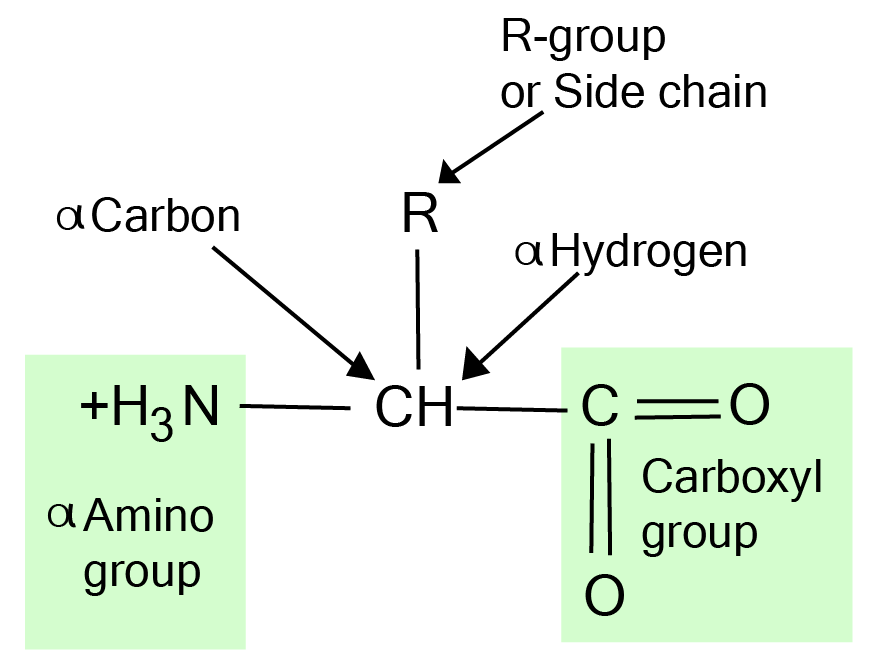
Here, the central (α) carbon is attached to:
An amino group (–NH₂)
A carboxyl group (–COOH)
A hydrogen atom
A unique side chain
20 Amino Acids List with Chemical Formulas
Below is the amino acids list of the 20 amino acids found in proteins, along with their chemical formulas:
IMPORTANT INFORMATION - Across all known life forms, there are 22 amino acids encoded by genes. The standard genetic code incorporates 20 of these, while two additional amino acids—selenocysteine and pyrrolysine—are introduced through specialised translation mechanisms.
Sources of Amino Acids
Amino acids are vital to many bodily functions, and they must be obtained from various food sources, especially the essential amino acids which the body cannot produce. Key sources include:
Plant-Based Foods: Broccoli, beans, beetroots, pumpkin, cabbage, nuts, dry fruits, chia seeds, oats, peas, carrots, cucumber, green leafy vegetables, onions, soybeans, whole grains, peanuts, legumes, and lentils.
Fruits: Apples, bananas, berries, figs, grapes, melons, oranges, papaya, pineapple, and pomegranates.
Animal Products: Dairy products, eggs, seafood, chicken, meat, and pork.
Functions of Amino Acids
Amino acids perform a host of roles in the body:
Functions of Essential Amino Acids
Phenylalanine: Crucial for nervous system health and memory enhancement.
Valine: Promotes muscle growth and repair.
Threonine: Supports immune system function.
Tryptophan: Contributes to the production of vitamin B3 and serotonin—a hormone that regulates mood, sleep, and appetite.
Isoleucine: Aids in haemoglobin formation and insulin synthesis.
Methionine: Important for skin health and detoxification.
Leucine: Stimulates protein synthesis and growth hormone release.
Lysine: Essential for antibody, hormone, and enzyme production, as well as calcium absorption.
Histidine: Vital for the production of red and white blood cells.
Functions of Non-Essential Amino Acids
Alanine: Helps in detoxification and glucose production.
Cysteine: Acts as an antioxidant and aids collagen synthesis.
Glutamine: Supports brain function and nucleic acid synthesis.
Glycine: Assists cell growth and wound healing.
Glutamic Acid: Functions as a neurotransmitter and supports brain health.
Arginine: Promotes protein synthesis and immune function.
Tyrosine: Involved in thyroid hormone production and melanin synthesis.
Serine: Supports muscle growth and immune protein synthesis.
Asparagine: Facilitates nitrogen transport and DNA synthesis.
Aspartic Acid: Plays a role in metabolism and the synthesis of other amino acids.
Proline: Important for collagen formation and tissue repair.
Deficiency of Amino Acids
A deficiency in amino acids—whether from a lack of essential amino acids or imbalances in non-essential amino acids—can lead to various health issues. Symptoms may include:
Edema
Anaemia
Insomnia
Diarrhoea
Depression
Hypoglycaemia
Loss of appetite
Liver fat accumulation
Skin and hair problems
Headache, weakness, irritability, and fatigue
Interesting Facts & Real-World Applications
Here are some unique insights that set this guide apart:
Sports Nutrition: Many athletes focus on a balanced intake of essential and non-essential amino acids to aid muscle repair and enhance performance.
Medical Research: Recent studies are exploring how specific amino acid structure modifications can lead to innovative therapies for metabolic disorders.
Daily Life: Beyond their role in proteins, amino acids are also used in cosmetics and skincare formulations due to their role in collagen production and skin elasticity.
Further Links:


FAQs on Complete Guide to 20 Amino Acids: Types, Structures & Significance
1. What is an amino acid and why is it called a 'building block of life'?
An amino acid is an organic compound containing both an amino group (–NH₂) and a carboxyl group (–COOH). They are called the 'building blocks of life' because they link together in long chains to form proteins. Every protein in the body, which carries out essential functions for growth, repair, and metabolism, is constructed from these amino acid units.
2. What is the general structure of an amino acid?
Every amino acid shares a fundamental structure based on a central carbon atom, known as the alpha-carbon. This carbon is bonded to four different groups:
- An amino group (–NH₂)
- A carboxyl group (–COOH)
- A hydrogen atom (–H)
- A unique side chain, known as the R-group
3. What makes each of the 20 standard amino acids unique from one another?
The uniqueness of each amino acid comes from its variable side chain (the R-group). While the central carbon, amino group, carboxyl group, and hydrogen atom are common to all amino acids, the R-group is different for each one. This side chain determines the amino acid's specific properties, such as its size, polarity, and charge, which in turn dictates how it interacts and contributes to a protein's final three-dimensional structure and function.
4. How are amino acids primarily classified based on nutritional requirements?
Based on the body's ability to produce them, amino acids are classified into two main nutritional types:
- Essential Amino Acids: These cannot be synthesised by the human body and must be obtained from the diet. The nine essential amino acids are Histidine, Isoleucine, Leucine, Lysine, Methionine, Phenylalanine, Threonine, Tryptophan, and Valine.
- Non-Essential Amino Acids: These can be produced by the human body, so they are not required in the diet. Examples include Alanine, Asparagine, Aspartic acid, and Glutamic acid.
5. Besides nutrition, what are the other ways to classify amino acids?
Amino acids can also be classified based on the chemical properties of their R-group, which is crucial for understanding protein structure. The main chemical classifications include:
- Nonpolar, aliphatic R-groups: These are hydrophobic (e.g., Glycine, Alanine, Valine).
- Polar, uncharged R-groups: These are hydrophilic and can form hydrogen bonds (e.g., Serine, Threonine, Cysteine).
- Aromatic R-groups: These have aromatic rings in their side chains (e.g., Phenylalanine, Tyrosine, Tryptophan).
- Positively charged (basic) R-groups: They are hydrophilic (e.g., Lysine, Arginine, Histidine).
- Negatively charged (acidic) R-groups: They are hydrophilic (e.g., Aspartate, Glutamate).
6. What are zwitterions, and how do they relate to amino acids?
A zwitterion is a molecule that has separate positively and negatively charged groups, but an overall neutral charge. In aqueous solutions at a neutral pH, amino acids exist as zwitterions. The amino group (–NH₂) gains a proton to become –NH₃⁺, and the carboxyl group (–COOH) loses a proton to become –COO⁻. This dual-ion nature explains key properties of amino acids, such as their high melting points and solubility in water.
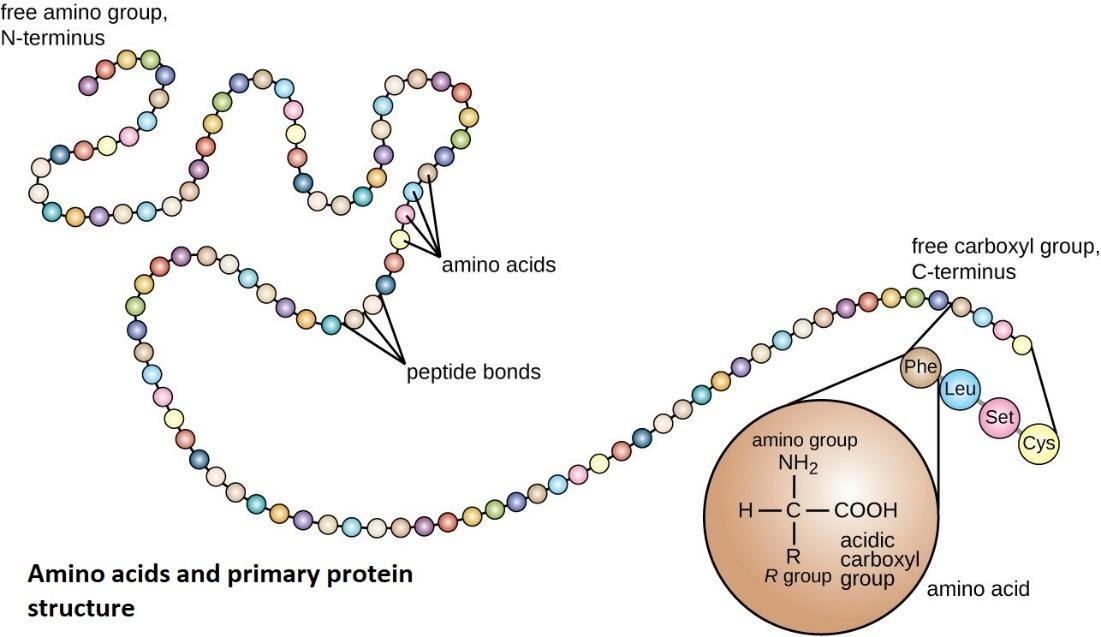
7. What is the difference between an amino acid, a peptide, and a protein?
These terms describe the hierarchy of protein structure:
- Amino Acid: The simplest, single monomer unit.
- Peptide: A chain formed when two or more amino acids are linked by peptide bonds. A short chain is an oligopeptide, while a long chain is a polypeptide.
- Protein: A functional biological molecule made of one or more polypeptide chains that have been folded into a specific, complex 3D shape, which is essential for its biological activity.
8. What are some key functions of amino acids in the body besides building proteins?
Beyond their primary role in protein synthesis, amino acids are vital for many other metabolic processes. For example, some amino acids act as precursors for other important molecules:
- Tryptophan is used to produce the neurotransmitter serotonin and the vitamin B3.
- Tyrosine is essential for synthesising thyroid hormones and melanin.
- Glutamic acid functions directly as a neurotransmitter in the brain.
- Arginine is involved in immune function and detoxification processes.
9. Are there really only 20 amino acids?
While 20 are considered the standard amino acids encoded by the universal genetic code to build proteins, there are more. The two other genetically encoded amino acids are selenocysteine and pyrrolysine, sometimes referred to as the 21st and 22nd amino acids. They are incorporated into proteins in some organisms through unique synthesis mechanisms that are not part of the standard set.










