
What is Crystal Field Theory?
Answer
490.2k+ views
Hint: The interaction of a metal ion and a ligand is treated as a purely electrostatic process in crystal field theory, which treats the ligands as point charges in the region of the central atom's atomic orbitals. The partially covalent character of bonding between the ligand and metal atom was taken into account in the development and expansion of crystal field theory, primarily through the use of molecular orbital theory. Ligand field theory is another name for crystal field theory.
Complete answer:
The net change in crystal energy caused by the orientation of d orbitals of a transition metal cation inside a coordinating group of anions, commonly known as ligands, is described by crystal field theory. Transition metals are known for their proclivity for forming complexes. A complex is made up of a core metal atom or ion that is surrounded by a number of ligands. Crystal field theory governs the interaction of these ligands with the core metal atom or ion.
Knowledge of the geometrical or spatial distribution of d orbitals is required to fully comprehend crystal field interactions in transition metal complexes. In a free gaseous metal ion, the d-orbitals are five fold degenerate. When a spherically symmetric field of negative ligand filled charge is applied to a central metal ion, the d-orbitals remain degenerate, but the energy of the free ion changes.
The interactions are summarised in the table below.
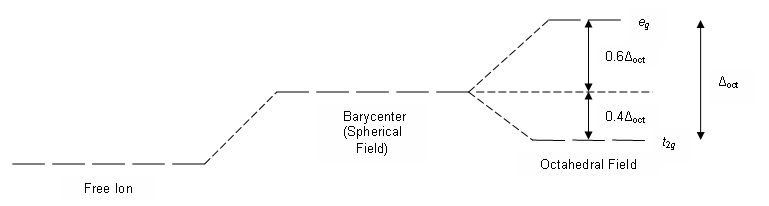
The metal-ligand bond was characterised as an ionic bond emerging only from electrostatic interactions between metal ions and ligands in the crystal field theory. Anions are treated as point charges in crystal field theory, while neutral molecules are treated as dipoles.
Transition metals' d orbitals are degenerate, meaning they have the same energy when they are not bound to any ligand. When they start connecting with other ligands, the d orbitals split apart and become non-degenerate due to various symmetries of the d orbitals and the inductive influence of the ligands on the electrons.
Note:
The following elements have an impact on this splitting:
-The metal ion's properties
-The oxidation state of the metal In comparison to the spherical field, a greater oxidation state causes more splitting.
-The ligands' arrangement around the metal ion
-The nature of the ligands around the metal ion (i.e. tetrahedral, octahedral...) the coordination number of the metal (i.e. tetrahedral, octahedral...) The larger the difference between the high and low energy d groups, the stronger the impact of the ligands.
Complete answer:
The net change in crystal energy caused by the orientation of d orbitals of a transition metal cation inside a coordinating group of anions, commonly known as ligands, is described by crystal field theory. Transition metals are known for their proclivity for forming complexes. A complex is made up of a core metal atom or ion that is surrounded by a number of ligands. Crystal field theory governs the interaction of these ligands with the core metal atom or ion.
Knowledge of the geometrical or spatial distribution of d orbitals is required to fully comprehend crystal field interactions in transition metal complexes. In a free gaseous metal ion, the d-orbitals are five fold degenerate. When a spherically symmetric field of negative ligand filled charge is applied to a central metal ion, the d-orbitals remain degenerate, but the energy of the free ion changes.
The interactions are summarised in the table below.

The metal-ligand bond was characterised as an ionic bond emerging only from electrostatic interactions between metal ions and ligands in the crystal field theory. Anions are treated as point charges in crystal field theory, while neutral molecules are treated as dipoles.
Transition metals' d orbitals are degenerate, meaning they have the same energy when they are not bound to any ligand. When they start connecting with other ligands, the d orbitals split apart and become non-degenerate due to various symmetries of the d orbitals and the inductive influence of the ligands on the electrons.
Note:
The following elements have an impact on this splitting:
-The metal ion's properties
-The oxidation state of the metal In comparison to the spherical field, a greater oxidation state causes more splitting.
-The ligands' arrangement around the metal ion
-The nature of the ligands around the metal ion (i.e. tetrahedral, octahedral...) the coordination number of the metal (i.e. tetrahedral, octahedral...) The larger the difference between the high and low energy d groups, the stronger the impact of the ligands.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




