
The calorific values of fuels A, B, C, and D are $48\, KJ/g$, $17\,KJ/g$, $50\, KJ/g$, $30\,KJ/g$, respectively. Which of these fuels could be wood?
1) A
2) B
3) C
4) D
Answer
588k+ views
Hint: The amount of heat energy produced on the complete burning of one kilogram of fuel in the presence of pure oxygen is called the calorific value of the substance. For woody biomass, the calorific value lies in between $17-21 KJ/g$, on a dry basis. Here $KJ$ denotes kilo joule and $g$ denotes gram. Now, this point has given us a clear vision of our solution.
Complete step by step answer:
Step 1:
Calorific value is the amount of heat energy present in food or fuel and which is determined by the complete combustion of a specified quantity at constant pressure and in normal conditions. It is also called calorific power. The unit of the calorific value is kilojoule per kilogram i.e. $KJ/g$.
Step 2:
The formula to calculate the calorific value used is the Net Calorific Value (NCV) = Gross Calorific Value (GCV)-Latent heat of water vapors.
Oven dry wood biomass typically has a calorific value equals to $17-21 MJ$ per kg. Here $MJ$ implies megajoule. The calorific value of wood is $17KJ/g$, but its value lies between $17-21KJ/g$ because most fuels are not oven dry when burnt and the water in the wood must be evaporated, detracting from the extractable energy.
To understand more about how moisture content in fuel changes the calorific value of a substance, have a look into the diagram below:
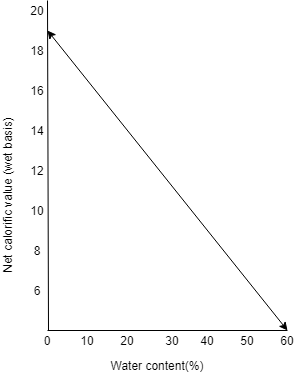
With the increase of water content in fuel, the net calorific value decreases. Here in the shown diagram on the Y-axis we have net calorific value (wet basis) in $KJ/g$ and in X-axis we have the water content in percentage when the water content for wood is zero then the calorific value is above the $18 KJ/g$ but with the increase in wetness, the value declines.
So, from the above diagram and statement, we can say that wood has a calorific value of $17 KJ/g$.
$\therefore $Option (2) is the correct answer.
Note:
- Calorific values are important in daily life because it is very important to have knowledge of the fuel to carry out day-to-day activities. This knowledge helps us to determine the amount of energy we transport.
- Through the use of calorific value, we can determine which fuel to be considered so as to maximize the use of energy with the blend of different types of fuels together.
- Natural gas has the highest calorific value at $13\, Kcal/g$, and also known for the cleanest fuel and coal has the lowest calorific value.
Complete step by step answer:
Step 1:
Calorific value is the amount of heat energy present in food or fuel and which is determined by the complete combustion of a specified quantity at constant pressure and in normal conditions. It is also called calorific power. The unit of the calorific value is kilojoule per kilogram i.e. $KJ/g$.
Step 2:
The formula to calculate the calorific value used is the Net Calorific Value (NCV) = Gross Calorific Value (GCV)-Latent heat of water vapors.
Oven dry wood biomass typically has a calorific value equals to $17-21 MJ$ per kg. Here $MJ$ implies megajoule. The calorific value of wood is $17KJ/g$, but its value lies between $17-21KJ/g$ because most fuels are not oven dry when burnt and the water in the wood must be evaporated, detracting from the extractable energy.
To understand more about how moisture content in fuel changes the calorific value of a substance, have a look into the diagram below:

With the increase of water content in fuel, the net calorific value decreases. Here in the shown diagram on the Y-axis we have net calorific value (wet basis) in $KJ/g$ and in X-axis we have the water content in percentage when the water content for wood is zero then the calorific value is above the $18 KJ/g$ but with the increase in wetness, the value declines.
So, from the above diagram and statement, we can say that wood has a calorific value of $17 KJ/g$.
$\therefore $Option (2) is the correct answer.
Note:
- Calorific values are important in daily life because it is very important to have knowledge of the fuel to carry out day-to-day activities. This knowledge helps us to determine the amount of energy we transport.
- Through the use of calorific value, we can determine which fuel to be considered so as to maximize the use of energy with the blend of different types of fuels together.
- Natural gas has the highest calorific value at $13\, Kcal/g$, and also known for the cleanest fuel and coal has the lowest calorific value.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




