
Smallest possible alkane with ethyl group as substituent possess molecular mass:
A. 16g
B. 72g
C. 84g
D. 100g
Answer
582.3k+ views
Hint:
Various ethyl substituted alkane can be drawn and then using IUPAC rule their name can be determined. Molecular mass of the alkane is the sum of the atomic mass of all carbon atoms and all hydrogen atoms.
Step by step answer: The general formula of alkane is as follows:
${{\text{C}}_{\text{n}}}{{\text{H}}_{{\text{2n + 2}}}}$
$n$ is the number of carbon atoms.
Ethyl substituents is represented as \[\left( {{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}} - } \right)\] .
Simplest alkane will be of one carbon that is methane ${\text{C}}{{\text{H}}_{\text{4}}}$ according to the alkane formula.
Ethyl substituents itself have two carbon atoms, so start with alkane having three carbon atoms that is propane.
The ethyl substituted propane is represented as follows:
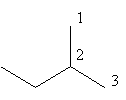
The above structure cannot ethyl substituted propane because according to IUPAC the longest carbon chain in this compound is of four carbon so, the actual name of the compound is $2 - $methyl butane so, alkane with three carbon cannot have ethyl substitution.
Check the next four carbon alkane that is butane. The ethyl substituted propane is represented as follows:
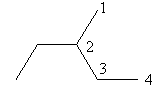
The above structure cannot ethyl substituted butane because according to IUPAC the longest carbon chain in this compound is of five carbon so, the actual name of the compound is $3 - $methyl pentane so, alkane with four carbon cannot have ethyl substitution.
Check the next five carbon alkane that is pentane. The ethyl substitution at carbon$ - 2$in pentane is represented as follows:
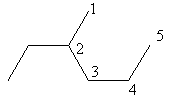
The above structure cannot ethyl substituted pentane because according to IUPAC the longest carbon chain in this compound is of six carbon so, the actual name of the compound is $3 - $methyl hexane so, pentane cannot have ethyl substitution at carbon$ - 2$.
Check the ethyl substitution at carbonin pentane is represented as follows:
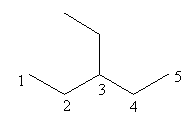
The above structure has ethyl substituted pentane and according to IUPAC the longest carbon chain in this compound is of five carbon so, the name of the compound is $3 - $ethyl pentane so, pentane cannot have ethyl substitution at carbon$ - 3$.
So, the smallest possible alkane with the ethyl substituted group is $3 - $ethyl pentane.
Determine the molecular mass of $3 - $ethyl pentane as follows:
The atomic mass of carbon is $12\,{\text{g}}$ and hydrogen is $1.0\,{\text{g}}$.
Mass No.$ = \,\left( {7\, \times \,12\,{\text{g}}} \right)\, + \,\left( {16\, \times \,1\,{\text{g}}} \right)$
Mass No. $ = \,100\,{\text{g}}$
So, the molecular mass of $3 - $ethyl pentane is $100\,{\text{g}}$.
Therefore, option (D) $100\,{\text{g}}$is correct.
Note: Ethane, propane and butane cannot have ethyl group as substituent. They can have methyl groups as substituents. Methane cannot have both methyl as well as ethyl as substituent.
Various ethyl substituted alkane can be drawn and then using IUPAC rule their name can be determined. Molecular mass of the alkane is the sum of the atomic mass of all carbon atoms and all hydrogen atoms.
Step by step answer: The general formula of alkane is as follows:
${{\text{C}}_{\text{n}}}{{\text{H}}_{{\text{2n + 2}}}}$
$n$ is the number of carbon atoms.
Ethyl substituents is represented as \[\left( {{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}} - } \right)\] .
Simplest alkane will be of one carbon that is methane ${\text{C}}{{\text{H}}_{\text{4}}}$ according to the alkane formula.
Ethyl substituents itself have two carbon atoms, so start with alkane having three carbon atoms that is propane.
The ethyl substituted propane is represented as follows:

The above structure cannot ethyl substituted propane because according to IUPAC the longest carbon chain in this compound is of four carbon so, the actual name of the compound is $2 - $methyl butane so, alkane with three carbon cannot have ethyl substitution.
Check the next four carbon alkane that is butane. The ethyl substituted propane is represented as follows:

The above structure cannot ethyl substituted butane because according to IUPAC the longest carbon chain in this compound is of five carbon so, the actual name of the compound is $3 - $methyl pentane so, alkane with four carbon cannot have ethyl substitution.
Check the next five carbon alkane that is pentane. The ethyl substitution at carbon$ - 2$in pentane is represented as follows:

The above structure cannot ethyl substituted pentane because according to IUPAC the longest carbon chain in this compound is of six carbon so, the actual name of the compound is $3 - $methyl hexane so, pentane cannot have ethyl substitution at carbon$ - 2$.
Check the ethyl substitution at carbonin pentane is represented as follows:

The above structure has ethyl substituted pentane and according to IUPAC the longest carbon chain in this compound is of five carbon so, the name of the compound is $3 - $ethyl pentane so, pentane cannot have ethyl substitution at carbon$ - 3$.
So, the smallest possible alkane with the ethyl substituted group is $3 - $ethyl pentane.
Determine the molecular mass of $3 - $ethyl pentane as follows:
The atomic mass of carbon is $12\,{\text{g}}$ and hydrogen is $1.0\,{\text{g}}$.
Mass No.$ = \,\left( {7\, \times \,12\,{\text{g}}} \right)\, + \,\left( {16\, \times \,1\,{\text{g}}} \right)$
Mass No. $ = \,100\,{\text{g}}$
So, the molecular mass of $3 - $ethyl pentane is $100\,{\text{g}}$.
Therefore, option (D) $100\,{\text{g}}$is correct.
Note: Ethane, propane and butane cannot have ethyl group as substituent. They can have methyl groups as substituents. Methane cannot have both methyl as well as ethyl as substituent.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




