
Lewis dot symbol indicates the number of electrons of the inner shell.
A. True
B. False
Answer
533.1k+ views
Hint: In the Lewis dot structure or symbol, the symbol of the element is written and there are dots arranged around it. For example, the atomic number of nitrogen is 7 and the numbers of dots written around the nitrogen symbol are 5, the atomic number of oxygen is 8 and the numbers of dots written around the oxygen symbol are 6, etc.
Complete answer:
We can tell many properties of the element by seeing the Lewis dot structure of the element. In the Lewis dot structure or symbol, the symbol of the element is written and there are dots arranged around it. So, the number of dots is equal to the number of electrons present in the valence shell, as the valence shell is the last shell in the respective atom.
For example, the atomic number of nitrogen is 7, which means there are 7 electrons in the nitrogen atom. So let us do the configuration:
$1{{s}^{2}}2{{s}^{2}}2{{p}^{3}}$
As we can see that in this the valence shell is 2 and there are two orbitals of the second shell and the total number of electrons in the valence shell is 5, therefore, the number of dots written around the nitrogen atom is 5.
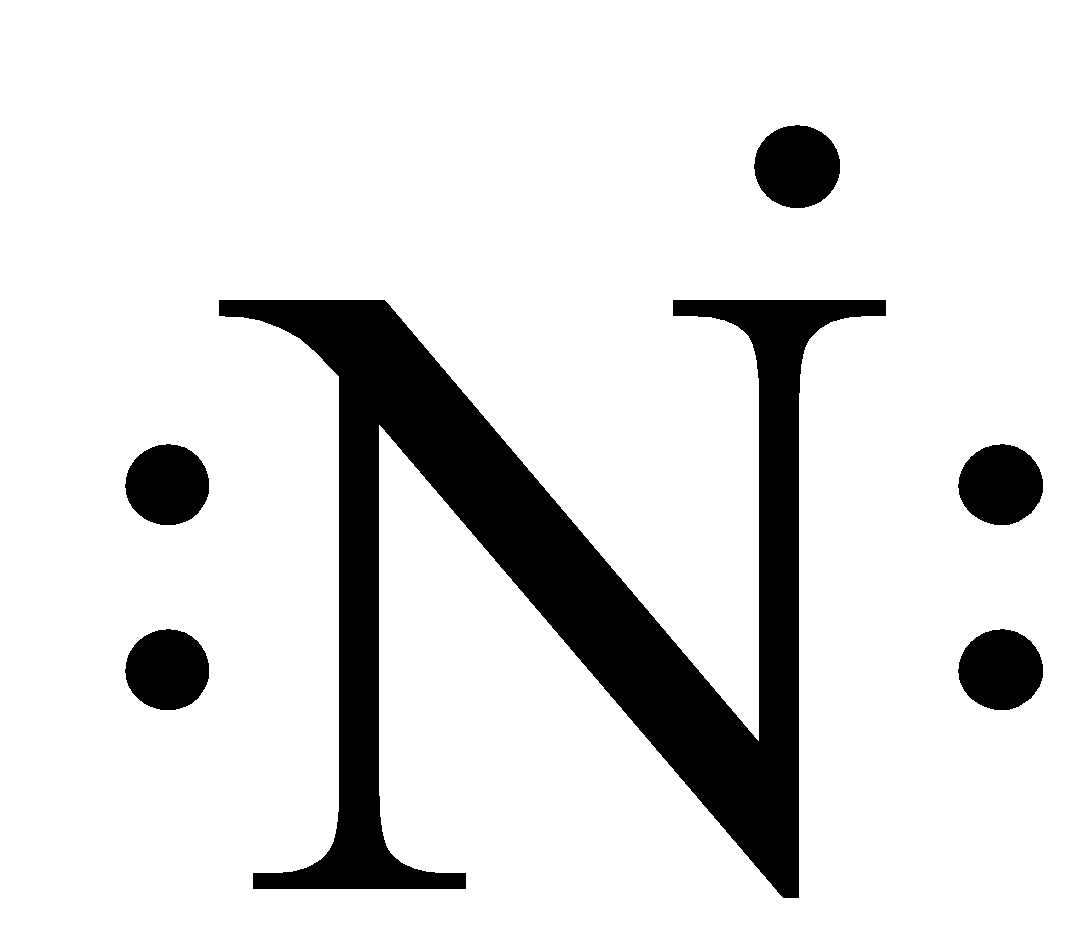
So, the electrons of the inner shell are not considered.
Another example is the atomic number of oxygen is 8, which means there are 8 electrons in the oxygen atom. So let us do the configuration:
$1{{s}^{2}}2{{s}^{2}}2{{p}^{4}}$
As we can see that in this the valence shell is 2 and there are two orbitals of the second shell and the total number of electrons in the valence shell is 6, therefore, the number of dots written around the oxygen atom is 6.
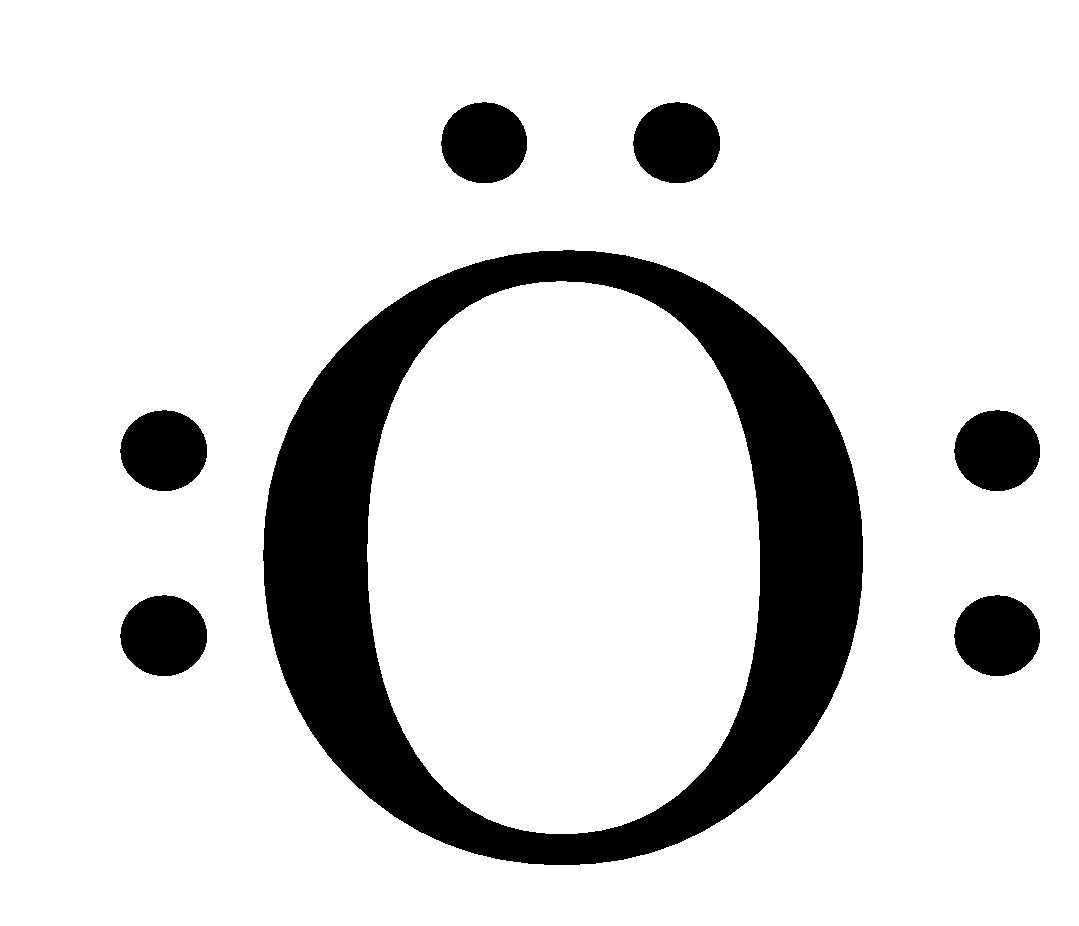
Therefore, the given statement in the question is false.
Note:
The number of electrons is equal to the number of electrons of all the orbitals of the valence shell, if the orbitals of the valence shell are 3 like s, p, and d then we have to count all the electrons in these orbitals.
Complete answer:
We can tell many properties of the element by seeing the Lewis dot structure of the element. In the Lewis dot structure or symbol, the symbol of the element is written and there are dots arranged around it. So, the number of dots is equal to the number of electrons present in the valence shell, as the valence shell is the last shell in the respective atom.
For example, the atomic number of nitrogen is 7, which means there are 7 electrons in the nitrogen atom. So let us do the configuration:
$1{{s}^{2}}2{{s}^{2}}2{{p}^{3}}$
As we can see that in this the valence shell is 2 and there are two orbitals of the second shell and the total number of electrons in the valence shell is 5, therefore, the number of dots written around the nitrogen atom is 5.
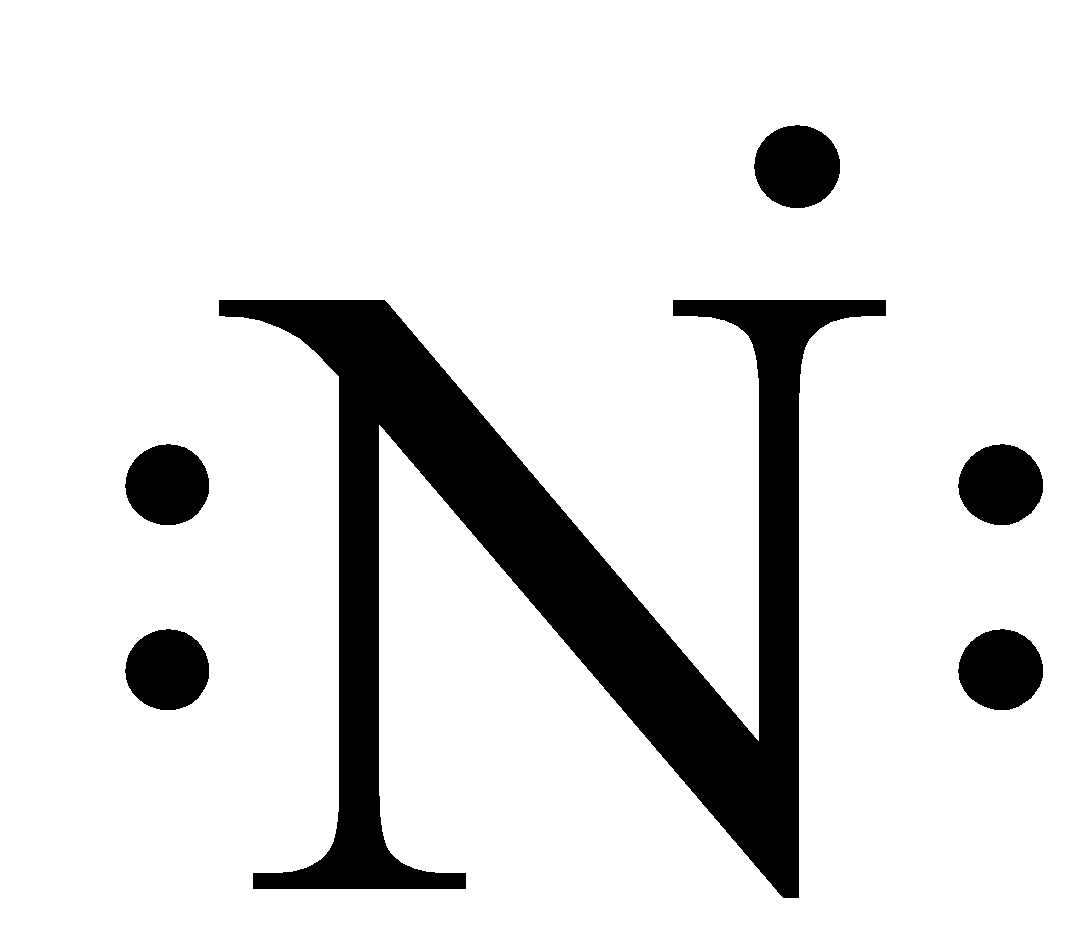
So, the electrons of the inner shell are not considered.
Another example is the atomic number of oxygen is 8, which means there are 8 electrons in the oxygen atom. So let us do the configuration:
$1{{s}^{2}}2{{s}^{2}}2{{p}^{4}}$
As we can see that in this the valence shell is 2 and there are two orbitals of the second shell and the total number of electrons in the valence shell is 6, therefore, the number of dots written around the oxygen atom is 6.

Therefore, the given statement in the question is false.
Note:
The number of electrons is equal to the number of electrons of all the orbitals of the valence shell, if the orbitals of the valence shell are 3 like s, p, and d then we have to count all the electrons in these orbitals.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




