
What does the melting point of a solid indicate?
Answer
580.8k+ views
Hint: Melting point of substance is temperature at which it melts or the temperature at which solid is converted into liquid.
Example: Melting point of ice is ${0^\circ }C$ it means at ${0^\circ }C$ ice converted into water.
Complete answer:
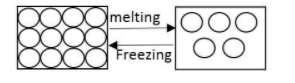
As we know at melting point substance changes state from solid to liquid.
$solid\overset {m.p.} \leftrightarrows liquid$
At melting point, the solid and liquid phase exist in equilibrium.
The melting point of a substance depends on pressure and temperature.
During the melting process the temperature of substance does not change. For example when ice melts to water the temperature of the system remains ${0^\circ }C$ throughout the change.
Melting point of some substances are
Melting point of compound determined by the force of attraction between molecules. If intermolecular attraction is greater than it results in higher melting point. Ionic compounds usually have a high melting point because the electrostatic force of attraction between them is very strong.
Note: The term ‘Freezing point’ is opposite of the term melting point.
It is used to denote the temperature at which a liquid is converted into solid.
Substances can be cooled below freezing points formation of solid. Such liquids are known as supercooled liquid.
Example: Melting point of ice is ${0^\circ }C$ it means at ${0^\circ }C$ ice converted into water.
Complete answer:
As we know at melting point substance changes state from solid to liquid.
$solid\overset {m.p.} \leftrightarrows liquid$
At melting point, the solid and liquid phase exist in equilibrium.
The melting point of a substance depends on pressure and temperature.
During the melting process the temperature of substance does not change. For example when ice melts to water the temperature of the system remains ${0^\circ }C$ throughout the change.
Melting point of some substances are
| Copper | 1359K |
| Aluminum | 932K |
| Hydrogen | 13.8K |
| Mercury | 234K |
Melting point of compound determined by the force of attraction between molecules. If intermolecular attraction is greater than it results in higher melting point. Ionic compounds usually have a high melting point because the electrostatic force of attraction between them is very strong.
Note: The term ‘Freezing point’ is opposite of the term melting point.
It is used to denote the temperature at which a liquid is converted into solid.
Substances can be cooled below freezing points formation of solid. Such liquids are known as supercooled liquid.

Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




