
Define or explain the following term.
Precipitation:
(A) Diffusion in a solid
(B) Reaction occurs in the gaseous medium
(C) Creation of solid in a solution
(D) None of these.
Answer
585k+ views
Hint: Precipitation is the process by which a solid is formed in a solution. After the solution is kept at rest for some time, the particles combine and form the solid substrate, precipitant.
Complete step by step solution:
-Discussing Option A, it is said that precipitation is diffusion in a solid. Let us first what diffusion is. The spreading out and mixing of a substance with another substance due to the motion of its particles is called diffusion. The diffusion of one substance into another substance goes on until a uniform mixture is formed. Precipitation is a diffusion in a solid.
-Discussing Option B, it is said that precipitation is a reaction that occurs in a gaseous medium. But, we know that precipitation occurs in a liquid medium. It needs that amount of force of gravity to settle down in the beaker. Until and unless it receives those it floats in the liquid medium as suspension. Precipitation occurs in a liquid medium.
-Discussing Option C, there is solid created in a solution. This solid thus formed after the settlement of particles is called precipitant. The precipitation may occur if the concentration of a compound exceeds its solubility (such as when mixing solvents or changing their temperature). Precipitation may also occur rapidly from a supersaturated solution.
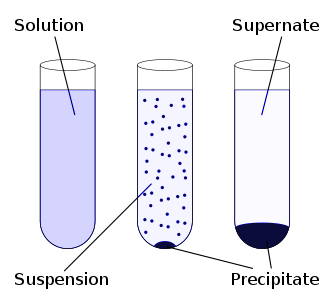
The correct answers are (A) and (C).
Note: Sometimes the formation of a precipitate indicates the occurrence of a chemical reaction. If the silver nitrate solution is poured into a solution of sodium chloride, a chemical reaction occurs forming a white precipitate of silver chloride. When potassium iodide solution reacts with lead(II) nitrate solution, a yellow precipitate of lead(II) iodide is formed.
Complete step by step solution:
-Discussing Option A, it is said that precipitation is diffusion in a solid. Let us first what diffusion is. The spreading out and mixing of a substance with another substance due to the motion of its particles is called diffusion. The diffusion of one substance into another substance goes on until a uniform mixture is formed. Precipitation is a diffusion in a solid.
-Discussing Option B, it is said that precipitation is a reaction that occurs in a gaseous medium. But, we know that precipitation occurs in a liquid medium. It needs that amount of force of gravity to settle down in the beaker. Until and unless it receives those it floats in the liquid medium as suspension. Precipitation occurs in a liquid medium.
-Discussing Option C, there is solid created in a solution. This solid thus formed after the settlement of particles is called precipitant. The precipitation may occur if the concentration of a compound exceeds its solubility (such as when mixing solvents or changing their temperature). Precipitation may also occur rapidly from a supersaturated solution.

The correct answers are (A) and (C).
Note: Sometimes the formation of a precipitate indicates the occurrence of a chemical reaction. If the silver nitrate solution is poured into a solution of sodium chloride, a chemical reaction occurs forming a white precipitate of silver chloride. When potassium iodide solution reacts with lead(II) nitrate solution, a yellow precipitate of lead(II) iodide is formed.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

State and prove converse of BPT Basic Proportionality class 10 maths CBSE




