
What are the periodic trends for atomic radii, ionization energy, and electron affinity?
Answer
494.4k+ views
Hint: According to the Modern Periodic Law given by Moseley, “The chemical and physical properties of the elements are periodic functions of their atomic numbers.” As we know that in the periodic table, the elements are arranged according to their atomic number. Hence, the chemical and physical properties of the elements depend upon their atomic numbers.
Complete answer:
To explain this question, we have to divide it into three parts.
Part-1: Atomic Radii
Variation of atomic radii in the periodic table:
Variation along a period:
On moving from left to right in the period, the atomic radius decreases as nuclear charge increases in each succeeding element by one unit while the number of the shells remains unchanged. Due to this escalated nuclear charge, the electrons of all the shells are pulled little closer to the nucleus making each shell smaller and smaller. By which the atomic radius decreases from moving left to right in the period.
\[Variation{\text{ }}of{\text{ }}atomic{\text{ }}radius{\text{ }}with{\text{ }}atomic{\text{ }}number{\text{ }}across{\text{ }}the{\text{ }}second{\text{ }}period:\]
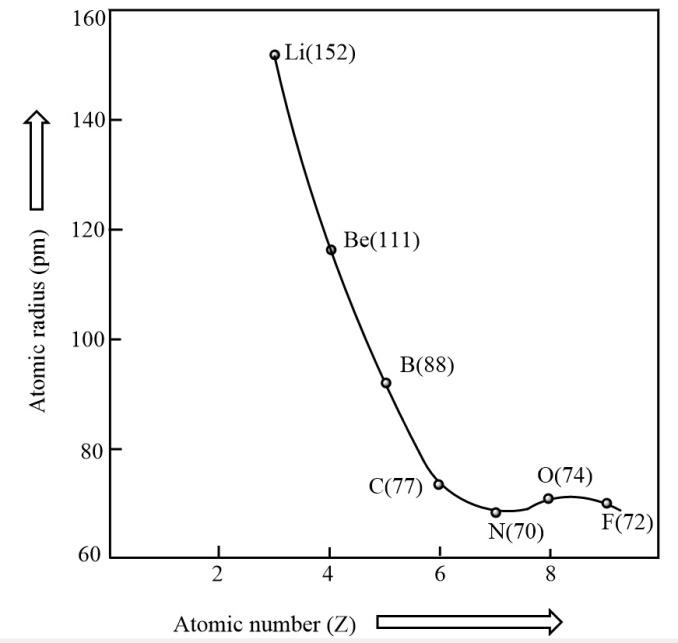
Variation along a group:
On moving down the group, the atomic radius increases as a new energy shell is added to each succeeding element and the valence electrons lie farther away from the nucleus. Hence, the attraction of the nucleus toward electrons decreases and thus the atomic radius increases.
Part-2: Ionization Energy
Variation of ionization energy in the periodic table:
Variation along a period:
On moving from left to right in the periodic table, the ionization energy of an element increases as the atomic radius decreases and nuclear charge increases in each succeeding element by one unit while the number of the shells remains unchanged. Hence increases the force of attraction for which more energy is required for the removal of electrons. Thus, ionization enthalpy increases.
Variation along a group:
On moving down the group, the ionization energy of an element decreases as the atomic radius increases due to the addition of a new energy shell in each succeeding element. By which the force of attraction decreases and less amount of energy is required for the removal of electrons. Thus, ionization enthalpy decreases.
Part-3: Electron Affinity
Variation of electron affinity in the periodic table:
Variation along a period:
On moving from left to right in the periodic table, the electron affinity of an element increases as the atomic radius decreases. Hence the force of attraction becomes stronger which causes the electrons to move closer to the nucleus. Thus, electron affinity increases.
Variation along a group:
On moving down the group, the electron affinity of an element decreases as the atomic radius increases due to the addition of a new energy shell in each succeeding element by which electrons further away from the nucleus. With increasing distance between the negatively charged electron and the positively charged nucleus, the force of attraction decreases hence electron affinity decreases.
Note:
Periodic trends are the illustration of different aspects of a certain element which includes its size and its electronic properties. Periodic trends are formed because of the arrangement of the periodic table which provides chemists a useful tool to predict electronic properties of the element very quickly.
Complete answer:
To explain this question, we have to divide it into three parts.
Part-1: Atomic Radii
Variation of atomic radii in the periodic table:
Variation along a period:
On moving from left to right in the period, the atomic radius decreases as nuclear charge increases in each succeeding element by one unit while the number of the shells remains unchanged. Due to this escalated nuclear charge, the electrons of all the shells are pulled little closer to the nucleus making each shell smaller and smaller. By which the atomic radius decreases from moving left to right in the period.
\[Variation{\text{ }}of{\text{ }}atomic{\text{ }}radius{\text{ }}with{\text{ }}atomic{\text{ }}number{\text{ }}across{\text{ }}the{\text{ }}second{\text{ }}period:\]

Variation along a group:
On moving down the group, the atomic radius increases as a new energy shell is added to each succeeding element and the valence electrons lie farther away from the nucleus. Hence, the attraction of the nucleus toward electrons decreases and thus the atomic radius increases.
Part-2: Ionization Energy
Variation of ionization energy in the periodic table:
Variation along a period:
On moving from left to right in the periodic table, the ionization energy of an element increases as the atomic radius decreases and nuclear charge increases in each succeeding element by one unit while the number of the shells remains unchanged. Hence increases the force of attraction for which more energy is required for the removal of electrons. Thus, ionization enthalpy increases.
Variation along a group:
On moving down the group, the ionization energy of an element decreases as the atomic radius increases due to the addition of a new energy shell in each succeeding element. By which the force of attraction decreases and less amount of energy is required for the removal of electrons. Thus, ionization enthalpy decreases.
Part-3: Electron Affinity
Variation of electron affinity in the periodic table:
Variation along a period:
On moving from left to right in the periodic table, the electron affinity of an element increases as the atomic radius decreases. Hence the force of attraction becomes stronger which causes the electrons to move closer to the nucleus. Thus, electron affinity increases.
Variation along a group:
On moving down the group, the electron affinity of an element decreases as the atomic radius increases due to the addition of a new energy shell in each succeeding element by which electrons further away from the nucleus. With increasing distance between the negatively charged electron and the positively charged nucleus, the force of attraction decreases hence electron affinity decreases.
Note:
Periodic trends are the illustration of different aspects of a certain element which includes its size and its electronic properties. Periodic trends are formed because of the arrangement of the periodic table which provides chemists a useful tool to predict electronic properties of the element very quickly.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




