
Anthracene is purified through which of the following method:
A. Filtration
B. Crystallization
C. Distillation
D. Sublimation
Answer
567k+ views
Hint: Anthracene is polycyclic aromatic hydrocarbon whose molecular formula is ${C_{14}}{H_{10}}$ . It consists of three fused benzene rings. It is a crystalline and combustible solid and has a very weak aromatic odor. It is present abundantly.
Complete step by step answer:
Anthracene is polycyclic aromatic hydrocarbon.
Its molecular formula is ${C_{14}}{H_{10}}$ .
It consists of three fused benzene rings. The structure of anthracene is given below:
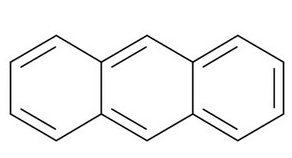
It is crystalline in nature and is a combustible solid which means that it can catch fire easily and also burn easily.
It is present in all types of coal and tar.
There are various methods involved to purify aromatic compounds namely: a) Filtration b) Crystallization c) Distillation d) Sublimation. We will see what each method means.
A.Filtration
Filtration is the process in which solid particles are separated from liquid.
The liquid which is separated from solid is called filtrate..
The filtration process takes place using a separating funnel.
B.Crystallization
Crystallization is the process in which a chemical is converted from a liquid into its solid crystalline form.
C.Distillation
distillation is the process in which liquid-liquid or solid-liquid mixtures are separated through selective boiling and condensing processes.
D.Sublimation
Sublimation is a process in which a solid component is converted into a gaseous form without any intermediate liquid phase. In simple words it means that the solid directly gets converted to gaseous form without passing through liquid form.
So basically of all these methods, the only method by which anthracene is purified is through the method of sublimation.
Anthracene is solid and volatile in nature.
Due to its volatile nature it easily gets converted from solid to gaseous form.
It is present in coal. So if you burn coal it directly gets converted from solid to gas.
So, the correct answer is Option D.
Note: Anthracene is three membered ring structure and whereas naphthalene is a two membered ring structure. Anthracene has three benzene rings and naphthalene has two benzene rings. They both are aromatic in nature.
Complete step by step answer:
Anthracene is polycyclic aromatic hydrocarbon.
Its molecular formula is ${C_{14}}{H_{10}}$ .
It consists of three fused benzene rings. The structure of anthracene is given below:

It is crystalline in nature and is a combustible solid which means that it can catch fire easily and also burn easily.
It is present in all types of coal and tar.
There are various methods involved to purify aromatic compounds namely: a) Filtration b) Crystallization c) Distillation d) Sublimation. We will see what each method means.
A.Filtration
Filtration is the process in which solid particles are separated from liquid.
The liquid which is separated from solid is called filtrate..
The filtration process takes place using a separating funnel.
B.Crystallization
Crystallization is the process in which a chemical is converted from a liquid into its solid crystalline form.
C.Distillation
distillation is the process in which liquid-liquid or solid-liquid mixtures are separated through selective boiling and condensing processes.
D.Sublimation
Sublimation is a process in which a solid component is converted into a gaseous form without any intermediate liquid phase. In simple words it means that the solid directly gets converted to gaseous form without passing through liquid form.
So basically of all these methods, the only method by which anthracene is purified is through the method of sublimation.
Anthracene is solid and volatile in nature.
Due to its volatile nature it easily gets converted from solid to gaseous form.
It is present in coal. So if you burn coal it directly gets converted from solid to gas.
So, the correct answer is Option D.
Note: Anthracene is three membered ring structure and whereas naphthalene is a two membered ring structure. Anthracene has three benzene rings and naphthalene has two benzene rings. They both are aromatic in nature.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

State and prove converse of BPT Basic Proportionality class 10 maths CBSE




