




An Overview of Class 12 Chemistry Chromatography Experiment
Introduction
Chromatography is critical in ensuring the safety of pharmaceuticals. Chromatography is used by pharmaceutical companies to quantify and analyse compounds for contaminants. Because their atoms differ slightly in space, chiral compounds have two distinct forms. One type of chiral compound has been shown to be toxic. Chromatography can ensure that the safe form of the chiral compound is distinct from the dangerous form. Vaccination development is another application of chromatography. Chromatography can be used to identify which antibodies are most effective at fighting and neutralising specific diseases.
Liquid chromatography, in conjunction with mass spectrometry, has transformed clinical laboratory testing. While mass spectrometry can identify analytes based on two physical properties, precursor and product ion mass, when used in conjunction with liquid chromatography, another property is added to identify the analyte even more precisely. Multiplexing, or the ability to identify and quantify multiple analytes at the same time, is also provided by liquid chromatography and mass spectrometry. This significantly reduces the amount of time and money spent on clinical trials.
Table of Contents
Aim of the experiment
Apparatus required
Theory
Procedure
Observations
Result
Precautions
Aim of the Experiment
To separate the components using chromatography technique.
Apparatus Required
Chromatography Jar
Thin-layer Chromatography Plate
Capillary Tube
Mobile Phase
Stationary Phase
Theory
The basic principle of chromatography is that molecules in a mixture are applied to the surface or into a solid, and the fluid stationary phase (stable phase) separates from each moving mobile phase. You should also assume that two spots in the final chromatogram that are the same colour and have travelled the same distance up the paper are most likely the same compound.
Procedure
Take a dry and clean chromatographic jar.
A paper soaked in the mobile phase is applied to the jar's walls to ensure that the environment is saturated with solvent vapours.
Close the jar after adding a mobile phase.
Equilibrium should be Maintained.
Make a mark on the adsorbent to represent the baseline.
Apply the sample to the Thin Line Chromatography plate using a capillary tube and allow it to dry.
Close the jar and place the plates inside.
Don’t disturb the jar until solvent is moved.
Remove and dry the Thin Line Chromatography plate.
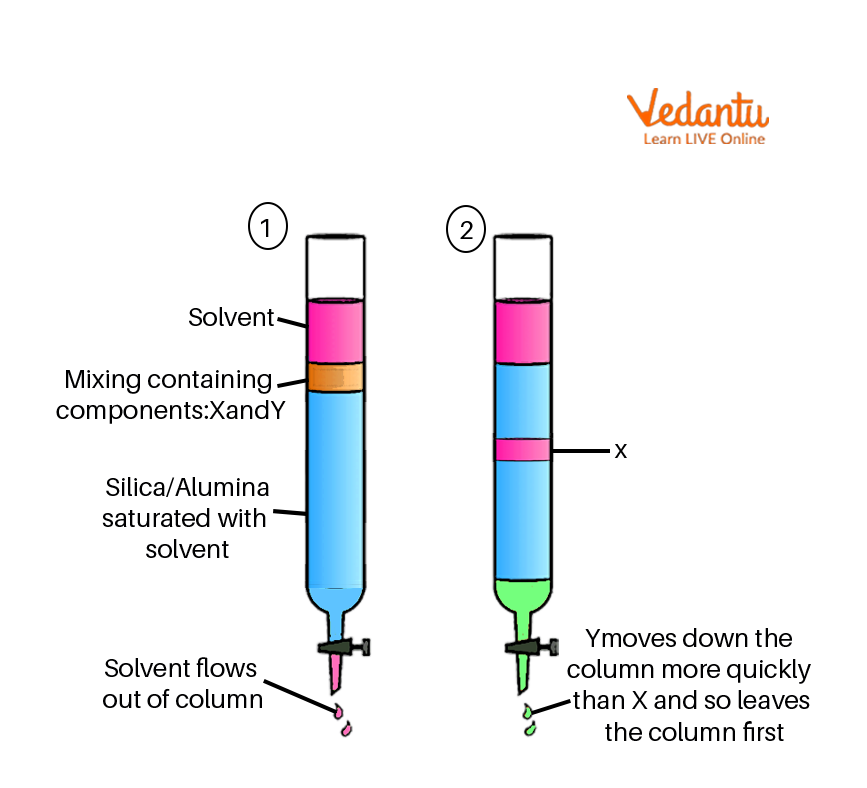
Chromatography diagram
Observations
Result
The mixture of two solvents gets separated at the end of the experiment.
Precautions
Before beginning the experiment, ensure the chromatographic jar is clean.
Allow the chromatographic jar to dry after cleaning it.
Maintain equilibrium throughout the experiment.
Lab Manual Questions
1. What is a chromatogram? Explain the principle of chromatography.
Ans. the pattern formed on an adsorbent medium by the layers of components separated by chromatography is called a chromatogram. Chromatography is based on the principle where molecules in a mixture are applied onto the surface or into the solid, and the fluid stationary phase (stable phase) separates from each other while moving with the aid of a mobile phase.
2. What are the essential characteristics of the substance to be used as a developer?
Ans.
It should be volatile.
It should impart colour to the different spots.
It should not react with various compounds which are being separated.
3. How is the phenomenon of ‘adsorption’ applied in the separation of compounds by chromatography technique?
Ans. Adsorption Chromatography involves separating a chemical mixture based on the interaction of the adsorbate with the adsorbent. In this process, the mixture of gas or liquid gets separated on the adsorbent bed, which adsorbs different compounds at different rates.
4. What are the types of chromatography?
Ans. Various chromatography methods have been developed to that end. Some include column chromatography, thin-layer chromatography (TLC), paper chromatography, gas chromatography, ion exchange chromatography, gel permeation chromatography, high-pressure liquid chromatography, and affinity chromatography.
Viva Questions
1. What is meant by the term Rf value in chromatography?
Ans. The retention factor (Rf) is used in thin-layer chromatography to compare and help identify compounds. The Rf value of a compound is equal to the distance travelled by the compound divided by the distance travelled by the solvent front (both measured from the origin).
2. Name the scientist who introduced the chromatographic technique.
Ans. Chromatography was first devised in Russia by the Italian-born scientist Mikhail Tsvet in 1900. He developed the technique and coined chromatography in the first decade of the 20th century, primarily for separating plant pigments such as chlorophyll, carotenes, and xanthophylls.
3. What are the benefits of chromatography over other techniques?
Ans. Precise separation, analyses, and purification are possible using chromatography. It requires shallow sample volumes. It works on a wide range of samples, including drugs, food particles, plastics, pesticides, air and water samples, and tissue extracts.
4. What are the disadvantages of Chromatography?
Ans. It is a time-consuming process for the separation of compounds. It is expensive as higher quantities of solvents are required. The automated process becomes complicated and, therefore, costly. It has a low separation power.
5. What is meant by the term developing in chromatography technique?
Ans. In terms of operation, in development chromatography, the mobile phase flow is stopped before solutes reach the end of the stationary phase bed. The mobile phase is called the developer and the movement of the liquid along the bed.
6. What are the benefits of Adsorption Chromatography?
Ans. The advantages of adsorption chromatography are as follows.
It has a wide range of mobile phases for separating compounds.
Adsorption chromatography is an important method to separate many components that are not separated by other techniques.
The complex sample mixtures can be easily separated by this method.
Very few apparatus or equipment types are required for isolation.
7. What are the disadvantages of Adsorption Chromatography?
Ans. The disadvantages of adsorption chromatography are as follows.
Obtained results from some methods of adsorption chromatography are complex to reproduce.
This can cause catalytic variations.
Some solutes take retention time to separate. This is a major disadvantage of adsorption chromatography.
It is more complicated and costly when making automation.
8. What is the main difference between adsorption and partition chromatography?
Ans. The fundamental differences between adsorption and partition chromatography are: Adsorption chromatography is used for solid-gas and solid-liquid chromatography. Whereas partition chromatography is the liquid–gas and liquid-liquid.
9. Can the Rf value be greater than 1?
Ans. By definition, Rf values are always less than 1. An Rf value of 1 or too close to it means that the spot and the solvent front travel close together and is therefore unreliable.
10. What can we conclude after seeing the rf value?
Ans. In chromatography, Rf values are the most basic prerequisite of the experiment. These numbers indicate whether the analyte (solute) prefers the stationary or mobile phase. With stationary and mobile phases, Rf values are used to determine polarity, relative masses, and relative solubilities, among other things.
Practical Based Questions
1. Which of the following is used as a spraying reagent in paper chromatography?
(a)Conc.HCl
(b)NaCl Solution
(c)Ninhydrin solution
(d)Cu Solution
Answer: C
2. In which type of chromatography, the stationary phase is held in a narrow tube and the mobile phase is forced through it under a narrow tube and the mobile phase is forced through it under pressure?
(a)Column Chromatography
(b)Planar chromatography
(c)Liquid chromatography
(d)Gas chromatography
Answer: A
3. Chromatography is a physical method that is used to separate
(a) Simple mixtures
(b) Complex mixtures
(c) Viscous mixtures
(d) Metals
Answer: B
4. Which force is involved in the Chromatography experiment?
(a) Hydrogen bonding
(b) London force
(c) Electric static force
(d) All of the above
Answer: D
5. Ion exchange chromatography is based on the
(a) Electrostatic attraction
(b) Electrical mobility of ionic species
(c) Adsorption chromatography
(d) Partition chromatography
Answer: A
6. Chromatography with solid stationary phase is called
(a) circle chromatography
(b) Square chromatography
(c) solid chromatography
(d) adsorption chromatography
Answer: D
7. What is the combination of paper chromatography and electrophoresis?
(a) Partition chromatography
(b) Electrical mobility of the ionic species
(c) Both (a) and (b)
(d) None of these
Answer: C
8. The pattern on the paper in chromatography is called
(a) chroming
(b) Chroma
(c) Chromatograph
(d) Chromatogram
Answer: D
9. In reverse phase chromatography, the stationary phase is made up of
(a) Non-polar
(b) Polar
(c) Both a and b
(d) None of these
Answer: A
10. In which chromatography stationary phase is more polar than mobile phase?
(a) Ion exchange chromatography
(b) Normal phase chromatography
(c) Reversed chromatography
(d) Size exclusion chromatography
Answer: B
Conclusion
Chromatography is the process of separating the components of a mixture by passing the solution or suspension of the mixture through a stationary medium in which the components move at different rates.
Chromatography is critical in ensuring the safety of pharmaceuticals. Pharmaceutical companies use chromatography to quantify and analyse compounds for contaminants.
The basic principle of chromatography is that molecules in a mixture are applied to the surface or into a solid. The fluid stationary phase (stable phase) separates from each moving mobile phase.
FAQs on Class 12 Chemistry Chromatography Experiment
1. What is the principle of chromatography and how is it important for Class 12 Chemistry board exams?
The principle of chromatography is based on the differential distribution of components in a mixture between a stationary phase and a mobile phase. In board exams, explaining how molecules separate due to their varying affinities toward these phases is critical for scoring in both conceptual and practical questions.
2. List the different types of chromatography that are commonly asked in CBSE Class 12 exams along with one key application for each.
Common types of chromatography and their applications include:
- Paper Chromatography – Separation of plant pigments
- Thin Layer Chromatography (TLC) – Checking drug purity
- Column Chromatography – Purification of chemicals
- Gas Chromatography – Analyzing volatile substances
- Ion Exchange Chromatography – Softening of hard water
- Gel Permeation Chromatography – Protein separation
3. How is the Rf value calculated in chromatography and why is it always less than 1?
The Rf value (Retention factor) is calculated by dividing the distance travelled by a component by the distance travelled by the solvent front from the origin. It is always less than 1 because the component can never move further than the solvent itself, which maintains the reliability of analysis.
4. What essential characteristics should a developer substance possess in a chromatography experiment to ensure top exam marks?
The developer substance should:
- Be volatile
- Impart distinct colours to different spots
- Not react with the compounds being separated
- Be able to efficiently develop and visualize the separated spots for evaluation
5. Explain the difference between adsorption chromatography and partition chromatography, a frequent 3-mark exam query.
Adsorption chromatography separates mixtures based on the adsorption of compounds on a solid surface, usually suitable for solid-gas or solid-liquid systems. Partition chromatography uses two liquid phases, with separation based on differing solubilities. Exam marks are awarded for both mechanism and example.
6. Why is chromatography preferred for separating and analyzing pharmaceutical compounds in board-level practical questions?
Chromatography is preferred because it provides high precision and accuracy in separating, identifying, and quantifying mixture components. It can detect contaminants and distinguish between active and toxic forms of compounds, ensuring pharmaceutical safety as required by Class 12 CBSE standards.
7. What practical precautions should students follow during chromatography experiments as per CBSE assessment guidelines?
Precautions include:
- Use a clean and dry chromatography jar
- Maintain a saturated atmosphere and equilibrium
- Apply the sample carefully at the marked baseline
- Do not disturb the setup during separation
- Allow proper drying of the chromatogram before analysis
8. What factors can affect the reproducibility and accuracy of results in a chromatography experiment for board exams?
Factors include:
- Layer thickness of stationary phase
- Amount of moisture on TLC/paper
- Temperature and vessel saturation
- Depth and nature of the mobile phase
- Sample size and spotting technique
9. Describe a common conceptual trap students should avoid when explaining the movement of components in chromatography for the exam.
A frequent mistake is assuming all spots that move the same distance are always different compounds. In reality, spots with the same color and Rf value are likely the same compound. Mislabeling can result in loss of marks; board exam answers must state this principle clearly.
10. How can mastering chromatography improve performance in Chemistry Olympiads and competitive exams alongside Class 12 board exams?
Mastering chromatography strengthens analytical skills in separation techniques, enhances understanding of molecular interactions, and enables students to tackle complex mixture analysis. These skills translate directly to higher-order thinking questions in Chemistry Olympiads, NEET, and JEE by providing a strong conceptual foundation beyond the NCERT curriculum.






































