How Do Plants Fix Nitrogen? Biological and Industrial Methods Explained
Nitrogen is one of the most abundant gases in our atmosphere, accounting for roughly 78% of the air we breathe. Despite its abundance in gaseous form, organisms cannot directly utilise atmospheric nitrogen for essential processes such as making proteins or nucleic acids. This is where nitrogen fixation comes into play. Through various pathways—including biological nitrogen fixation, lightning, and industrial nitrogen fixation—inert nitrogen gas (N₂) is transformed into more accessible forms like ammonia (NH₃) and nitrates. These compounds then become available to plants, and subsequently, to every link in the food chain.
In this comprehensive guide, you will learn about the different ways in which nitrogen fixation occurs, the mechanism of nitrogen fixation, the role of nitrogen fixation bacteria, and how nitrogen fixation in plants supports life on Earth. You will also discover additional facts about nitrogen metabolism, denitrification, and unique improvements you can explore in your study of this vital element.
Exploring the Forms and Pathways of Nitrogen Fixation
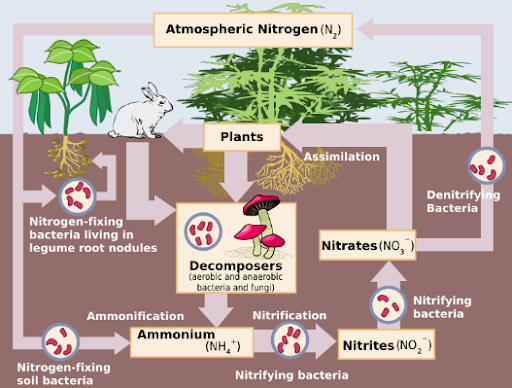
Nitrogen in the environment undergoes continuous recycling through the nitrogen cycle, a series of steps that ensures it is converted into usable forms and made available to living organisms. Below are the primary ways by which nitrogen fixation takes place:
1. Biological Nitrogen Fixation
Biological nitrogen fixation is carried out by specific prokaryotes (bacteria and some archaea) that possess the nitrogenase enzyme complex. This special enzyme converts atmospheric nitrogen (N₂) into ammonia (NH₃), which can then be taken up by plants.
Free-Living Bacteria: Some microbes, such as Azotobacter, Beijerinckia, Rhodospirillum, and various cyanobacteria, fix nitrogen independently in the soil or water.
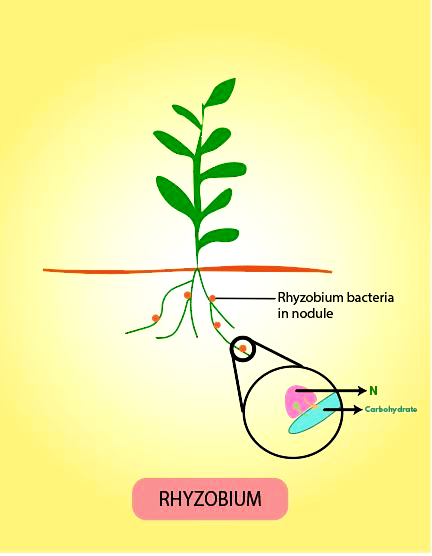
Symbiotic Bacteria: Certain nitrogen-fixation bacteria, such as Rhizobium and Frankia, form mutually beneficial (symbiotic) relationships with plants.
Rhizobium is commonly found in the root nodules of leguminous plants (e.g. peas, beans). Here, the plant supplies carbohydrates to the bacteria, while the bacteria convert atmospheric nitrogen into ammonia for the plant’s use.
Frankia forms nodules in non-leguminous plants like alder trees, carrying out a similar symbiotic function.
This natural mechanism of nitrogen fixation is crucial for agriculture, as it replenishes the soil with usable nitrogen. That is why crop rotation with leguminous plants is often practised to maintain soil fertility without excessive chemical fertilisers.
2. Nitrogen Fixation by Lightning
Have you ever wondered how a bolt of lightning can affect the soil? During thunderstorms, the extreme heat and pressure in the lightning pathway can break the triple bond of atmospheric nitrogen molecules. This process leads to the formation of nitrogen oxides (e.g., NO, NO₂). These gases dissolve in rainwater and eventually form nitrates in the soil, making them available for plant uptake. Although lightning contributes a relatively smaller portion of fixed nitrogen compared to biological nitrogen fixation, it plays a vital role in supplying nitrogen to areas with less agricultural activity.
3. Industrial Nitrogen Fixation
The industrial nitrogen fixation process, most famously represented by the Haber-Bosch method, revolutionised agriculture by producing ammonia on a large scale from nitrogen and hydrogen gases. This ammonia is then used to manufacture nitrogen-based fertilisers. Although this process helps meet the global food demand, it also requires high energy inputs and can have environmental impacts if not managed responsibly.
Mechanism of Nitrogen Fixation: How Does It Really Work?
When we talk about the mechanism of nitrogen fixation, the spotlight often falls on the nitrogenase enzyme. Found in nitrogen fixation bacteria, this enzyme has two main components:
Dinitrogenase Reductase – Transfers electrons from a reducing agent (often ferredoxin) to dinitrogenase.
Dinitrogenase – Uses these electrons to reduce atmospheric nitrogen (N₂) to ammonia (NH₃).
The reaction is energy-intensive, requiring significant ATP to break the strong triple bond in N₂. The simplified equation for the nitrogenase reaction is:
N₂ + 8e⁻ + 8H⁺ + 16ATP → 2NH₃ + H₂ + 16ADP + 16Pi
Once formed, ammonia (NH₃) can be quickly converted to ammonium ions (NH₄⁺) in the presence of water. In plants, this ammonium is utilised to synthesise amino acids and other biomolecules.
Nitrogen Fixation in Plants: Significance and Examples
Nitrogen fixation in plants is central to agriculture and ecosystem sustainability. Leguminous plants, such as peas, beans, lentils, and clover, are the most familiar examples. Their root nodules house nitrogen fixation bacteria like Rhizobium. However, non-leguminous plants like certain alder species (in association with Frankia) also tap into this beneficial partnership.
Benefits in Agriculture
Reduced Fertiliser Use: Growing legumes can naturally enrich the soil with nitrogen, reducing the need for chemical fertilisers.
Enhanced Soil Health: Crop rotation with legumes helps in preventing nutrient depletion and improves soil structure.
Sustainable Farming Practices: Encouraging biological nitrogen fixation aligns with eco-friendly approaches to farming, lowering the risk of soil and water pollution.
Nitrogen Metabolism and Denitrification
Once nitrogen is fixed into ammonia or nitrates, it becomes a part of plant and animal tissues. Eventually, these compounds return to the soil when organisms excrete waste or when they die and decompose. The next steps include:
Ammonification: Decomposition of organic matter into ammonia or ammonium.
Nitrification: Conversion of ammonium ions to nitrates (NO₃⁻) by nitrifying bacteria like Nitrosomonas and Nitrobacter.
Denitrification: Certain bacteria such as Pseudomonas and Clostridium convert nitrates back into atmospheric nitrogen (N₂), especially in oxygen-deprived environments.
This cyclical process ensures a steady balance of nitrogen in ecosystems. Any disruption can impact plant growth, crop yields, and broader ecological relationships.
Practical Applications
To ensure our content stands out, here are some additional points not commonly emphasised:
Urban Farming & Hydroponics: Even in controlled environments like hydroponic systems, nitrogen fixation remains vital. Growers often introduce beneficial bacteria or use fertilisers derived from industrial nitrogen fixation to maintain plant health.
Biofertilisers: Scientists are studying ways to harness advanced formulations of nitrogen fixation bacteria as biofertilisers to boost sustainable agriculture.
Biotechnology Innovations: Genetic engineering aims to introduce the genes required for biological nitrogen fixation into non-leguminous crops like wheat or maize. This could potentially reduce reliance on synthetic fertilisers and mitigate environmental pollution.
Interactive Quiz: Test Your Knowledge on Nitrogen Fixation
Which of the following gases is most abundant in Earth’s atmosphere?
A. Oxygen
B. Nitrogen
C. Carbon dioxide
D. Argon
What is the main enzyme complex involved in the mechanism of nitrogen fixation?
A. Nitrogenase
B. Amylase
C. Protease
D. Lipase
Name one free-living bacterium that carries out biological nitrogen fixation:
A. Rhizobium
B. Azotobacter
C. E. coli
D. Pseudomonas
Which process is responsible for converting nitrates back into nitrogen gas (N₂)?
A. Nitrification
B. Denitrification
C. Ammonification
D. Industrial nitrogen fixation
Which nitrogen-fixing bacteria is commonly found in root nodules of legumes?
A. Pseudomonas
B. Nitrobacter
C. Rhizobium
D. Spirillum
Check Your Answers
B
A
B
B
C


FAQs on Nitrogen Fixation and Metabolism: Student’s Complete Guide
1. What is nitrogen fixation, and why is this process considered fundamental to sustaining life on Earth?
Nitrogen fixation is the crucial process of converting atmospheric nitrogen (N₂), which is largely unusable by most organisms, into reactive nitrogen compounds like ammonia (NH₃). This process is fundamental because nitrogen is a core component of essential organic molecules, including amino acids (the building blocks of proteins) and nucleic acids (DNA and RNA). Without nitrogen fixation, the vast reservoir of atmospheric nitrogen would remain inaccessible, limiting the growth and survival of plants and, consequently, all other life forms in the ecosystem.
2. What are the main types of nitrogen fixation?
Nitrogen fixation occurs through three primary methods:
- Biological Nitrogen Fixation: This is carried out by specialised microorganisms, known as diazotrophs. Examples include symbiotic bacteria like Rhizobium in legume root nodules and free-living bacteria like Azotobacter.
- Atmospheric Nitrogen Fixation: This occurs naturally when the immense energy from lightning strikes breaks the triple bond of nitrogen molecules, allowing them to combine with oxygen. These nitrogen oxides then dissolve in rain, forming nitrates that enrich the soil.
- Industrial Nitrogen Fixation: This is a synthetic process, most notably the Haber-Bosch process, where atmospheric nitrogen and hydrogen are combined under high temperature and pressure to produce ammonia for fertilisers.
3. Why can't plants and animals use the nitrogen gas (N₂) directly from the atmosphere?
Most organisms cannot use atmospheric nitrogen (N₂) directly because the two nitrogen atoms in the molecule are joined by a very strong triple covalent bond. This bond makes the N₂ molecule chemically inert and extremely stable. An immense amount of energy is required to break this bond to convert the nitrogen into a reactive form. Only specific microorganisms with the enzyme nitrogenase or high-energy events like lightning can provide the necessary energy to break this bond.
4. How is symbiotic nitrogen fixation different from non-symbiotic nitrogen fixation?
The key difference lies in the relationship between the nitrogen-fixing microbe and a host plant.
- In symbiotic nitrogen fixation, the bacteria (e.g., Rhizobium) live in a mutually beneficial relationship with a host plant, typically forming nodules on its roots. The plant provides carbohydrates and a stable environment, while the bacteria provide fixed nitrogen directly to the plant.
- In non-symbiotic (free-living) nitrogen fixation, the microorganisms (e.g., Azotobacter, Clostridium) live independently in the soil and fix nitrogen for their own needs. When they die and decompose, the fixed nitrogen is released into the soil, becoming available to plants.
5. What is the specific role of the enzyme nitrogenase in biological nitrogen fixation, and why is it so sensitive?
The enzyme nitrogenase is the catalyst for biological nitrogen fixation. Its specific role is to break the strong triple bond of N₂ gas and reduce it to ammonia (NH₃). This reaction is highly energy-intensive, requiring a significant amount of ATP. Nitrogenase is extremely sensitive to oxygen and is irreversibly inactivated by it. To protect this enzyme, nitrogen-fixing organisms have developed various mechanisms, such as the oxygen-scavenging pigment leghaemoglobin found in the root nodules of legumes.
6. What is an example of nitrogen fixation that does not involve living organisms?
A primary example of non-biological nitrogen fixation is atmospheric fixation caused by lightning. The enormous energy released during a lightning strike provides enough power to break the triple bond of atmospheric nitrogen gas (N₂). These free nitrogen atoms can then react with oxygen (O₂) to form nitrogen oxides (NOx). These oxides dissolve in rainwater to form nitric acid, which falls to the earth and adds nitrates to the soil, making them available for plants.
7. How does the agricultural practice of crop rotation with legumes relate to nitrogen fixation?
Crop rotation with legumes like peas, beans, and clover is a sustainable agricultural practice directly related to symbiotic nitrogen fixation. Legumes host Rhizobium bacteria in their root nodules, which fix large amounts of atmospheric nitrogen into the soil. When a non-leguminous crop like wheat or corn is planted in the same field the following season, it benefits from the nitrogen-rich soil left behind by the legumes. This natural fertilisation reduces the need for synthetic nitrogen fertilisers, improves soil health, and promotes sustainable farming.
8. Can nitrogen fixation occur in plants other than legumes?
Yes, symbiotic nitrogen fixation is not exclusive to legumes. Certain non-leguminous plants, such as Alder (Alnus) and Casuarina, form symbiotic relationships with nitrogen-fixing bacteria of the genus Frankia. Similar to legumes, these plants develop root nodules where the bacteria reside and convert atmospheric nitrogen into a form the plant can use, allowing these plants to thrive in nitrogen-poor soils.
9. How does denitrification contrast with nitrogen fixation within the broader nitrogen cycle?
Denitrification and nitrogen fixation are two opposing but equally essential processes that balance the nitrogen cycle. While nitrogen fixation converts inert atmospheric nitrogen (N₂) into biologically available forms like ammonia, denitrification is the process where soil bacteria (e.g., Pseudomonas denitrificans) convert nitrates (NO₃⁻) back into nitrogen gas (N₂), which is then released into the atmosphere. Essentially, fixation removes nitrogen from the air and puts it into the ecosystem, while denitrification returns it to the air, completing the cycle.










