Types of Diffusion and Biological Examples
Diffusion is a core biological process that allows molecules to move passively from an area of higher concentration to one of lower concentration. It is essential for the natural movement of substances in and out of all living cells, helping organisms survive and function efficiently. Diffusion does not require cellular energy and takes place due to the random motion of particles, aiming to reach an even, balanced distribution (equilibrium) across a given space or membrane.
Diffusion: Meaning and Biological Importance
In biology, diffusion is the passive transport of molecules like gases, water, nutrients, and wastes. Molecules naturally move down their concentration gradient, from where they are more concentrated to where they are less concentrated, without any input of energy from the cell. This action is crucial for processes such as respiration, waste removal, and nutrient absorption in both plants and animals.
A simple daily-life example is dropping a soluble crystal (like sugar) in water. Over time, the sugar spreads evenly, coloring the water. This occurs because the sugar particles move from high to low concentration areas until they are evenly distributed.
Key Features of Diffusion in Cells
- Passive process: No ATP or cellular energy needed.
- Molecules move from high to low concentration.
- Random movement continues until equilibrium is achieved.
- Occurs in both liquids and gases, and especially across biological membranes.
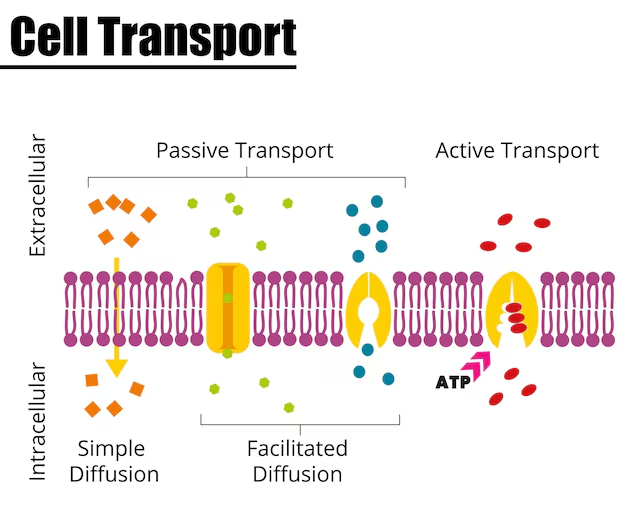
Types of Diffusion
Diffusion in biological systems is mainly classified into two types:
- Simple Diffusion: Molecules move directly through the cell membrane without aid. Small and uncharged molecules like oxygen, water, and carbon dioxide pass this way.
- Facilitated Diffusion: Larger or charged molecules (like glucose, ions) need special protein channels or carriers to cross the cell membrane. This process remains passive and uses no energy, but relies on specific protein helpers.
| Feature | Simple Diffusion | Facilitated Diffusion |
|---|---|---|
| Membrane Assistance | No Protein Needed | Requires Protein Channels/Carriers |
| Types of Molecules | Small, nonpolar (O2, CO2) | Large, polar/charged (Glucose, Ions) |
| Energy Requirement | No | No |
Factors Affecting Rate of Diffusion
- Temperature: Warmer temperatures increase molecular movement, speeding up diffusion.
- Surface Area: Greater surface area allows more molecules to pass through at once.
- Particle Size: Smaller particles move and diffuse faster than larger ones.
- Concentration Gradient: A bigger difference in concentration promotes a faster diffusion rate.
Diffusion in Everyday Life: Examples and Applications
- Tea Bag in Hot Water: Tea molecules diffuse into water, coloring it evenly.
- Perfume in a Room: Fragrance diffuses and spreads, allowing people at a distance to smell it.
- Sugar in Water: Even without stirring, sugar spreads due to molecular movement.
- Rehydration of Noodles: Water molecules diffuse into dried food, making it soft.
Diffusion vs Osmosis: Key Differences
| Aspect | Diffusion | Osmosis |
|---|---|---|
| What Moves? | All types of molecules (solute or solvent) | Only water molecules |
| Membrane Required? | Not always | Always (semipermeable membrane) |
| Direction | High to low concentration | Water moves from low solute concentration to high solute concentration |
Why Diffusion Alone Is Not Enough in Multicellular Organisms
- Distance Limitation: Diffusion is efficient only over short distances. In large organisms, it is too slow for deep tissues.
- Metabolic Demand: Complex tissues need faster delivery of gases and nutrients than diffusion alone can provide.
- Evolution of Transport Systems: Animals and plants have developed specialized circulatory and transport systems for efficient long-distance movement of substances.
Scientific Significance of Diffusion
- Essential for breathing, as oxygen and carbon dioxide gases diffuse in and out of lungs and blood.
- Helps in absorption of nutrients and excretion of waste in cells.
- Key for plant root uptake of water and minerals.
Practice Question
Q: Why does facilitated diffusion require membrane proteins, while simple diffusion does not?
Answer: Facilitated diffusion is for molecules too large or charged to cross the membrane directly. Proteins help them pass passively, whereas small, nonpolar molecules can move freely via simple diffusion.
Continue Learning
Diffusion remains a foundational concept for understanding life at the cellular and organism level. Grasping diffusion helps students connect core processes in cell biology, human physiology, and botany efficiently.


FAQs on Diffusion: Principle and Role in Cell Transport
1. What is diffusion in the context of biology?
Diffusion is the passive movement of molecules or ions from an area of higher concentration to an area of lower concentration, down a concentration gradient, until equilibrium is reached. This process does not require energy and is essential for many biological functions, such as gas exchange, nutrient absorption, and waste removal in living organisms.
2. What are the primary types of diffusion found in biological systems?
There are two main types of diffusion in biology:
• Simple diffusion: Movement of small, non-polar molecules (e.g., O2, CO2) directly through the cell membrane's lipid bilayer.
• Facilitated diffusion: Passive movement of large or charged molecules (e.g., glucose, ions) through specific carrier proteins or channels in the membrane.
3. Which factors affect the rate of diffusion across a membrane?
The rate of diffusion depends on:
• Concentration gradient (steeper gradient increases rate)
• Temperature (higher temperature increases kinetic energy and rate)
• Surface area (larger surface means faster diffusion)
• Particle size (smaller particles diffuse more rapidly)
• Membrane permeability (more permeable membranes allow faster passage)
4. What is the biological significance of diffusion for living organisms?
Diffusion is vital for survival because it enables cells to:
• Exchange gases such as oxygen and carbon dioxide
• Absorb nutrients from their surroundings
• Remove waste products efficiently
• Maintain essential concentration gradients and regulate cellular processes
5. Can you provide some common examples of diffusion in biology?
Examples of diffusion in biology include:
• Exchange of oxygen and carbon dioxide in lung alveoli
• Absorption of dissolved minerals and water by root hairs in plants
• Movement of neurotransmitters across synaptic gaps in nerve cells
6. How is diffusion different from osmosis, and why are both crucial for a cell?
Diffusion is the movement of any type of molecules from high to low concentration. Osmosis is a specific type of diffusion that involves water molecules moving across a semipermeable membrane from low solute concentration to high solute concentration. Both are crucial because diffusion manages transport of gases and nutrients, while osmosis balances water content, ensuring cell survival.
7. Why is simple diffusion not sufficient for the transport needs of large, multicellular organisms?
Simple diffusion is effective only over short distances. In larger organisms with complex body structures, many cells are located far from the external environment. As a result, simple diffusion is too slow to meet their metabolic requirements, necessitating specialized systems like the circulatory and respiratory systems for efficient transport.
8. How does a concentration gradient drive the process of facilitated diffusion?
In facilitated diffusion, molecules move passively from regions of higher concentration to lower concentration through specific membrane proteins. The concentration gradient serves as the driving force, allowing substances to move without energy input until equilibrium is reached on both sides of the membrane.
9. Does diffusion stop once equilibrium is reached?
Diffusion does not completely stop at equilibrium. Instead, there is no net movement of molecules—particles continue to move randomly in both directions at equal rates, maintaining a state known as dynamic equilibrium.
10. What is facilitated diffusion? Give an example.
Facilitated diffusion is a form of passive transport where molecules move across the cell membrane through specific carrier or channel proteins without using cellular energy (ATP).
Example: Movement of glucose into red blood cells via GLUT transporter proteins.
11. How is diffusion across the cell membrane important for respiration?
During respiration, oxygen diffuses from alveoli into blood, and carbon dioxide diffuses out of blood into alveoli due to differences in concentration gradients. This diffusion is essential for gas exchange and energy production in cells.
12. What is the difference between simple diffusion and active transport?
Simple diffusion is passive, moving molecules from high to low concentration without energy. Active transport requires ATP and can move molecules against their concentration gradient (from low to high).
Example: Simple diffusion—oxygen entering cells; Active transport—sodium-potassium pump in neurons.










